Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nutrition & Dietetics
Equol, produced from daidzein, is the isoflavone-derived metabolite with the greatest estrogenic and antioxidant activity. Consequently, equol has been endorsed as having many beneficial effects on human health. The conversion of daidzein into equol takes place in the intestine via the action of reductase enzymes belonging to minority members of the gut microbiota.
- equol
- daidzein
- isoflavones
- soy
- soy products
- gut metabolite
- bioactive compound
1. Introduction
Abundant epidemiological evidence suggests that diets rich in phytoestrogen-containing foods, such as soy and soy products, reduce the risk of a number of syndromes and chronic diseases, notably menopause symptoms in women, cardiovascular and neurodegenerative diseases, and certain types of cancer [1][2][3]. Isoflavones are non-nutritive phenolic compounds found in the roots and seeds of many plants, of these, soybeans are the richest source [4]. Soy isoflavones are phytoestrogens resembling 17-β-estradiol; although less active than the hormone, they show estrogen-like activity [5]. Extracted from soy or other sources, isoflavones can be administered as food supplements.
In plants, isoflavones are found mostly (>80%) in the form of glycoconjugates, i.e., the glucosides genistin, daidzin, and glycitin, and the corresponding acetyl and malonyl derivatives [6]. Glycosides are not readily absorbed in the gut and have only low-level estrogenic activity [7]. For isoflavones to become bioavailable and functional, these glycosides must be hydrolyzed into their corresponding isoflavone-aglycones, i.e., genistein, daidzein, and glycitein [8].
The amount of aglycones in plasma cannot be predicted from soy or isoflavone ingestion, as many intrinsic (genetic background, gut microbiota, bowel disease, age, sex, etc.) and extrinsic (isoflavone source, method of extraction, formulation, etc.) factors influence their bioavailability [9]. The plasma isoflavone concentration in humans is only 0.5–1.3% of that actually absorbed, much lower than in animal models (0.5–3.1% for rats and 3.1–26.0% in mice) [10]. Results obtained in animal studies may not, therefore, be easily extrapolated to humans. Absorbed aglycones are metabolized mainly to glucuronidated and sulphated derivatives by endogenous phase I and phase II enzymes [9][11]. They may then be further catabolized in the liver or secreted into the bile, thus returning to the intestine via the enterohepatic circulation [12].
Certain aglycone conjugates may have estrogenic activity too, and serve as an intracellular reservoir for the release of free aglycones in target cells [13]. However, as for absorption, extrapolation of plasma concentrations to the tissue level may not be valid. Indeed, the identification and quantification of the isoflavone derivatives in target tissues has rarely been determined [14]. In humans, equol concentrations have been reported to range between 22–36 and 456–559 nmol/kg in breast adipose and glandular tissue respectively [15][16].
Unabsorbed isoflavones and those excreted by the biliary system to the intestine reach the colon where they are deconjugated by bacterial enzymes and then (re)absorbed or further metabolized [12][17]. In the gut, isoflavone aglycones can be metabolized by intestinal microbes via several reactions, including reduction, methylation/demethylation, hydroxylation/dihydroxylation, and C-ring cleavage (Figure 1).
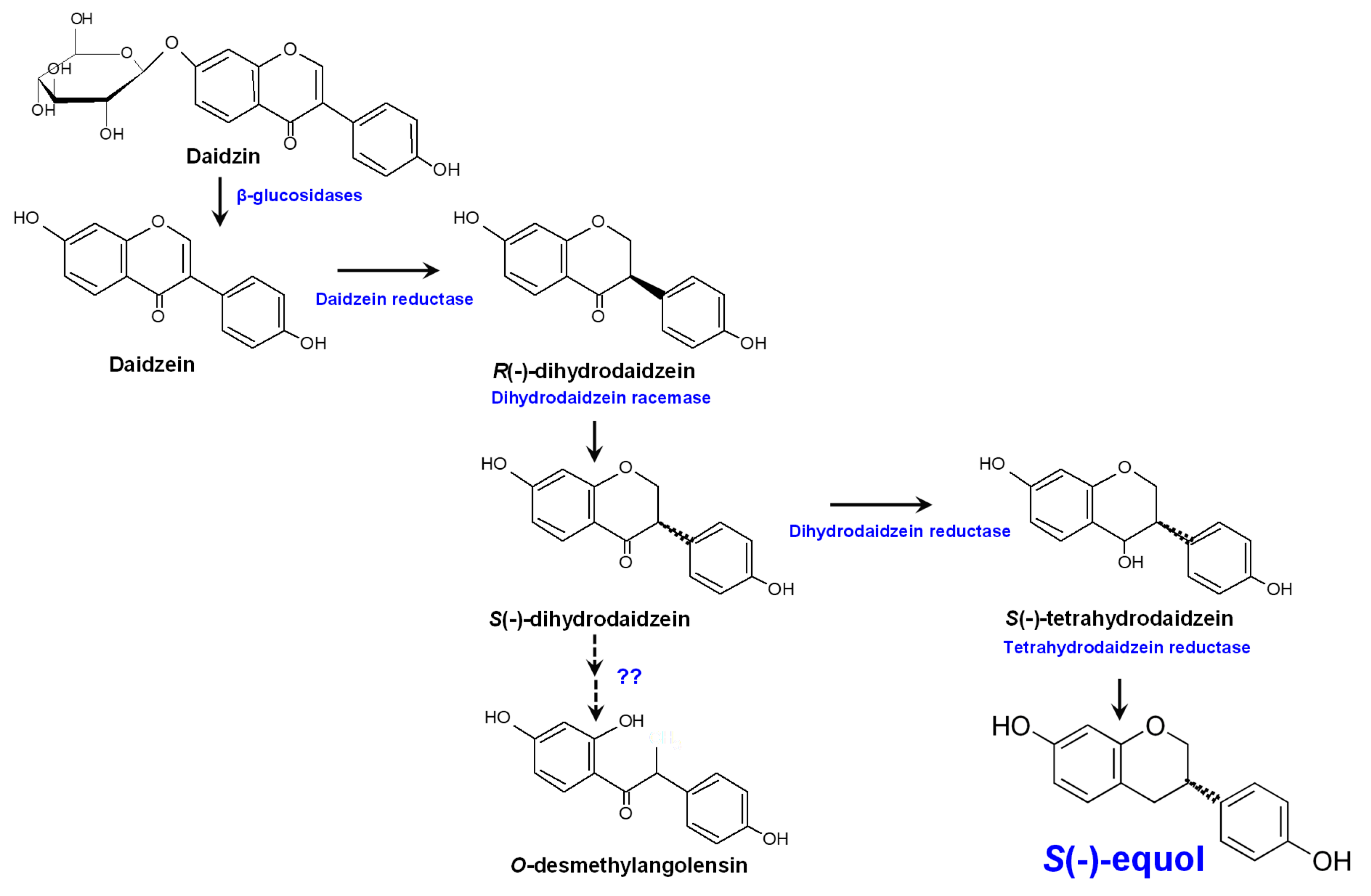
Figure 1. Metabolism of the isoflavone glucoside daidzein by the human gut microbiota and equol biosynthesis pathway.
2. Equol
Extracted from the urine of pregnant mares back in 1932, equol [C15H12O(OH)2] was the first isoflavonoid to be identified [18]. Later, in 1982 it was the first isoflavone-derived compound detected in human urine and blood (reviewed by Setchell and Clerici [19]). Equol is an isoflavone-derived metabolite formed from daidzin/daidzein by bacteria in the distal region of the small intestine and colon [19]. From a chemical viewpoint, equol (4’,7-isoflavandiol) is an isoflavane phenolic compound with a non-planar structure, which might be responsible for its physiological activities [20]. Equol is optically active with an asymmetric carbon atom at the C3 position giving rise to R(-)- and S(-)-equol enantiomers. However, only S(-)-equol has been detected as a result of bacterial daidzein conversion [21][22][23]. Equol is more stable, more easily absorbed, and has a lower clearance than its precursor molecule daidzein [24]. It also shows stronger estrogenic activity than any other isoflavone or isoflavone-derived metabolite [23][25]. As for other isoflavones, equol also shows anti-androgenic activity by binding to and sequestering 5α-dihydrotestosterone [26]. In addition, it is the isoflavone-derived compound with the strongest antioxidant activity [27][28]; antioxidants are thought to have a prominent role in the onset and progress of different chronic diseases, including cancer [29].
3. Soy, Soy Isoflavones, Equol, and Health
In East Asian countries, climacteric vasomotor symptoms during menopause in women are less severe than in Western women and the incidence of cardiovascular disease, osteoporosis, mental disorders, and certain types of cancer is about two- to four-fold of that seen in the West [1][2][3][30][31][32]. Alongside genetic factors, this large difference is assumed to have a nutritional basis. Isoflavones are an important component of Asian diets (15–50 mg day versus <2 mg in Western countries) [33][34][35][36], and observational and epidemiological studies have correlated a high intake of soy and isoflavones with reduced menopause symptoms, increased bone formation, reduced bone resorption, improved learning, and a reduced risk of prostate, colon, and breast cancer [37][38][39][40].
In vitro laboratory studies and animal interventions can predict the impact of isoflavones on human health, but only human trials can provide proof. However, most current human interventions involving isoflavones have suffered from small sample sizes, short trial durations, lack of appropriate controls, the use of isoflavones from various sources, supplements with different aglycone contents, and other methodological flaws [41]. Not surprisingly, this has led to inconsistent results being reported [31][42][43][44][45][46]. Indeed, most reviews and meta-analyses report the results of soy and isoflavone intervention studies to be far from conclusive [1][47][48][49][50][51][52]. As a result, regulatory agencies usually conclude there to be no scientifically sound evidence of isoflavones reducing the risks and symptoms of any disease [14][41][53]. In addition to the effect of genetic variation on the phenotypic expression of human disease [54][55], interpersonal differences in the intestinal microbiota may also account (at least in part) for the discrepancies seen [56][57]. Such differences could give rise to different microbial isoflavone-derived metabolites being produced [12][58], which might explain the lack of effectiveness in some studies. In particular, there has been much speculation regarding the reason why just a fraction (25 to 50%) of the human population produces equol. Conceivably, these subjects might be the only ones who would benefit from soy or isoflavone consumption [46][59][60]. To test this hypothesis, the categorization of the individuals in isoflavone trials by their equol-producing phenotype is pivotal. This only began in recent years [61][31][60][62][63] and no firm conclusions have yet been drawn. Indeed, the results of many studies have been very conflicting [43][44][45][46][64][65][66][67].
In contrast to their possible health benefits, the anti-estrogenic properties of isoflavones might also cause them to act as unwanted endocrine disruptors [68]. In vitro and animal studies both report isoflavones able to interfere with different checkpoints of the hypothalamic/pituitary/thyroid system [69]. This could have a huge repercussion on thyroid homeostasis. Further, the estrogenic activity of isoflavones (and thus equol) could pose a potential hazard by promoting certain types of tumor [70]. However, the scientific evidence supporting their having any harmful consequences is also inconclusive [14][41].
4. Mechanistic Mode of Action of Equol
The mechanistic mode of action of equol is not yet completely understood. Studies have mostly been done through in vitro assays using concentrations higher than those found under physiological conditions, thus limiting the provision of robust and definitive conclusions. Further, equol is found in plasma mainly as a 7-O-glucuronide derivative [71], which makes it difficult to discern the biologically-active form(s) at tissue and cellular levels. In spite of these deficits, evidence from experimental studies suggests that equol may act in multiple ways [72]. Based on its structural similarity to 17-β-estradiol, equol binds to both estrogen receptors (ERs) α (ERα) and β (ERβ—the preferred target) with greater affinity than its precursor daidzein, and to a degree comparable to that of genistein [73][74]. As ERs are not equally distributed among the different tissues, equol might have different effects depending on the ratio of ERα and ERβ isoforms present. Whether it acts as an agonist or an antagonist may further depend on the level of endogenous estrogens present, as they bind to both receptors more tightly [75]. The antioxidant activity of equol seems to be mostly mediated by its interaction with the ERβ [27], which induces the extracellular signal-regulated protein kinases (ERK1/2) and the NF-κB peptide, factors that control transcription, cytokine production, and cell survival [76]. Isoflavones and equol may not act as antioxidants themselves but rather by triggering cell signaling pathways leading to changes in the expression of cellular enzymes such as superoxide dismutase, catalase, and glutathione peroxidase (all involved in counteracting oxidative stress) [29]. Mechanistically, equol’s influence on transcription seems to proceed through the activation of the transactivation function AF-1 [77]. Another mode of action of equol underlying several physiological effects may relate to epigenetic mechanisms, including DNA methylation, histone modification, and microRNA regulation [78].
Equol has been reported to induce acute endothelium- and nitric oxide (NO)-dependent relaxation of the aortic rings, and is a potent activator of the human and mouse pregnane X receptor (PXR), a steroid and xenobiotic sensing protein in the nucleus [79]. Further, it has been proposed that it modulates endothelial redox signaling and NO release, involving the transactivation of the epidermal growth factor receptor kinase (EGFRK) and the reorganization of the F-actin cytoskeleton [80]. Equol has also been shown to prevent (at physiological concentrations) oxidized LDL-stimulated apoptosis in human umbilical vein endothelial cells [81], and to reduce the oxidative stress induced by lipopolysaccharides in chicken macrophages [82]. These activities may provide the basis for therapeutic strategies, for instance by restoring endothelial function in cardiovascular diseases. An improvement in atherosclerosis has also been reported via equol attenuating ER stress, mediated by the activation of the NF-E2 p45-related factor 2 (Nrf2) signaling pathway [83]. The cancer-protective effects of isoflavones and equol have been attributed to a variety of signaling pathways, including the regulation of the cell cycle (by reducing the activity of the cyclin B/CDK complex) [84], the inhibition of cell proliferation (by, for instance, reducing activity of topoisomerase II) [85], the induction of apoptosis [76][86][87], and the degradation of androgen receptor by S-phase kinase-associated protein 2 (PKAP2) [88]. The anti-prostate cancer activity of equol in cell cultures has been proposed associated with activation of FOXO3a (one of the forkhead-family factors of transcription involved in apoptosis) via protein kinase B (Akt)-specific signaling transduction pathway, and with the inhibition of the expression of the MDM2 complex (a negative regulator of tumor suppressor p53) [89][90], plus the inhibition of the degradation of the androgen receptor [88]. Diabetes and other metabolic disorders may be influenced by equol via its preventing glucagon-like peptide 1 (GLP-1) secretion from the GLUTag cells [91]. The modulation of glucose-induced insulin secretion and the suppression of glucagon release (from the α- and β-pancreatic cells, respectively) by GLP-1 in response to the ingestion of nutrients have been firmly established [92]. Equol can also prevent hypoglycemia by activating cAMP signaling at the plasma membrane of INS-1 pancreatic β-cells [93]. Finally, it has been reported to significantly increase the expression of genes coding for collagen, elastin, and tissue inhibitor of metalloproteases, while reducing the expression of metalloproteinases [94]. All these factors contribute towards an improvement of the skin’s antioxidant status, delaying aging. However, despite of all the knowledge gathered by these in vitro observations, the effects of equol on human health in vivo, and their magnitude, are yet to be confirmed.
This entry is adapted from the peer-reviewed paper 10.3390/nu11092231
References
- Bolaños, R.; Del Castillo, A.; Francia, J. Soy isoflavones versus placebo in the treatment of climacteric vasomotor symptoms: Systematic review and meta-analysis. Menopause 2010, 17, 660–666.
- Bilal, I.; Chowdhury, A.; Davidson, J.; Whitehead, S. Phytoestrogens and prevention of breast cancer: The contentious debate. World J. Clin. Oncol. 2014, 5, 705–712.
- Wada, K.; Nakamura, K.; Tamai, Y.; Tsuji, M.; Kawachi, T.; Hori, A.; Takeyama, N.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; et al. Soy isoflavone intake and breast cancer risk in Japan: From the Takayama study. Int. J. Cancer 2013, 133, 952–960.
- Aguiar, C.L.; Baptista, A.S.; Alencar, S.M.; Haddad, R.; Eberlin, M.N. Analysis of isoflavonoids from leguminous plant extracts by RPHPLC/DAD and electrospray ionization mass spectrometry. Int. J. Food Sci. Nutr. 2007, 58, 116–124.
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25.
- Kudou, S.; Fleury, Y.; Welti, D.; Magnolato, D.; Uchida, T.; Kitamura, K.; Okubo, K. Malonylisoflavone glycosides in soybean seeds (Glycine max Merrill). Agric. Biol. Chem. 1991, 51, 5579–5597.
- Shinkaruk, S.; Carreau, C.; Flouriot, G.; Bennetau-Pelissero, C.; Potier, M. Comparative effects of R- and S-equol and implication of transactivation functions (AF-1 and AF-2) in estrogen receptor-induced transcriptional activity. Nutrients 2010, 2, 340–354.
- Setchell, K.D.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001, 131, 1362S–1375S.
- Soukup, S.T.; Helppi, J.; Müller, D.R.; Zierau, O.; Watzl, B.; Vollmer, G.; Diel, P.; Bub, A.; Kulling, S.E. Phase II metabolism of the soy isoflavones genistein and daidzein in humans, rats and mice: A cross-species and sex comparison. Arch. Toxicol. 2016, 90, 1335–1347.
- Grace, P.B.; Taylor, J.I.; Low, Y.L.; Luben, R.N.; Mulligan, A.A.; Botting, N.P.; Dowsett, M.; Welch, A.A.; Khaw, K.T.; Wareham, N.J.; et al. Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European Prospective Investigation of Cancer and Nutrition-Norfolk. Cancer Epidemiol. Biomark. Prev. 2004, 13, 698–708.
- Kulling, S.E.; Lehmann, L.; Metzler, M. Oxidative metabolism and genotoxic potential of major isoflavone phytoestrogens. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 777, 211–218.
- Franke, A.A.; Lai, J.F.; Halm, B.M. Absorption, distribution, metabolism, and excretion of isoflavonoids after soy intake. Arch. Biochem. Biophys. 2014, 59, 24–28.
- Pugazhendhi, D.; Watson, K.A.; Mills, S.; Botting, N.; Pope, G.S.; Darbre, P.D. Effect of sulphation on the oestrogen agonist activity of the phytoestrogens genistein and daidzein in MCF-7 human breast cancer cells. J. Endocrinol. 2008, 197, 503–515.
- Hüser, S.; Guth, S.; Joost, H.G.; Soukup, S.T.; Köhrle, J.; Kreienbrock, L.; Diel, P.; Lachenmeier, D.W.; Eisenbrand, G.; Vollmer, G.; et al. Effects of isoflavones on breast tissue and the thyroid hormone system in humans: A comprehensive safety evaluation. Arch. Toxicol. 2018, 92, 2703–2748.
- Bolca, S.; Urpi-Sarda, M.; Blondeel, P.; Roche, N.; Vanhaecke, L.; Possemiers, S.; Al-Maharik, N.; Botting, N.; De Keukeleire, D.; Bracke, M.; et al. Disposition of soy isoflavones in normal human breast tissue. Am. J. Clin. Nutr. 2010, 91, 976–984.
- Maubach, J.; Depypere, H.T.; Goeman, J.; Van der Eycken, J.; Heyerick, A.; Bracke, M.E.; Blondeel, P.; De Keukeleire, D. Distribution of soy-derived phytoestrogens in human breast tissue and biological fluids. Obstet. Gynecol. 2004, 103, 892–898.
- Heinonen, S.; Hoikkala, A.; Wähälä, K.; Adlerreutz, H. Metabolism of the soy isoflavones daidzein, genistein and glycitein in human subjects. Identification of new metabolites having an intact isoflavonoid skeleton. J. Steroid Biochem. Mol. Biol. 2003, 87, 285–299.
- Marrian, G.F.; Haslewood, G.A. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares’ urine. Biochem. J. 1932, 26, 1227–1232.
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S.
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antiox. Redox Signal. 2013, 18, 1818–1892.
- Schwen, R.J.; Nguyen, L.; Charles, R.L. Elucidation of the metabolic pathway of S-equol in rat, monkey and man. Food Chem. Toxicol. 2012, 50, 2074–2083.
- Kim, M.; Kim, S.I.; Han, J.; Wang, X.L.; Song, D.G.; Kim, S.U. Stereospecific biotransformation of dihydrodaidzein into (3S)-equol by the human intestinal bacterium Eggerthella strain Julong 732. Appl. Environ. Microbiol. 2009, 75, 3062–3068.
- Setchell, K.D.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079.
- Setchell, K.D.; Faughnan, M.S.; Avades, T.; Zimmer-Nechemias, L.; Brown, N.M.; Wolfe, B.E.; Brashear, W.T.; Desai, P.; Oldfield, M.F.; Botting, N.P.; et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am. J. Clin. Nutr. 2003, 77, 411–419.
- Jackson, R.L.; Greiwe, J.S.; Schwen, R.J. Emerging evidence of the health benefits of S-equol, an estrogen receptor β agonist. Nutr. Rev. 2011, 69, 432–448.
- Lund, T.D.; Munson, D.J.; Haldy, M.E.; Setchell, K.D.; Lephart, E.D.; Handa, R.J. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol. Reprod. 2004, 70, 1188–1195.
- Choi, E.J.; Kim, G.H. The antioxidant activity of daidzein metabolites, O-desmethylangolensin and equol, in HepG2 cells. Mol. Med. Rep. 2014, 9, 328–332.
- Wei, X.J.; Wu, J.; Ni, Y.D.; Lu, L.Z.; Zhao, R.Q. Antioxidant effect of a phytoestrogen equol on cultured muscle cells of embryonic broilers. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 735–741.
- Alfa, H.H.; Arroo, R.R.J. Over 3 decades of research on dietary flavonoid antioxidants and cancer prevention: What have we achieved? Phytochem. Rev. 2019.
- Forman, D.; Ferlay, J. The global and regional burden of cancer. In World Cancer Report 2014; Stewart, B.W., Wild, C.P., Eds.; International Agency for Research on Cancer (IARC): Lyon, France, 2014; pp. 16–53.
- Liu, Z.M.; Ho, S.C.; Woo, J.; Chen, Y.M.; Wong, C. Randomized controlled trial of whole soy and isoflavone daidzein on menopausal symptoms in equol-producing Chinese postmenopausal women. Menopause 2014, 21, 653–660.
- He, F.J.; Chen, J.Q. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: Differences between Chinese women and women in Western countries and possible mechanisms. Food Sci. Hum. Wellness 2013, 2, 146–161.
- Yamori, Y. Food factors for atherosclerosis prevention: Asian perspective derived from analyses of worldwide dietary biomarkers. Exp. Clin. Cardiol. 2006, 11, 94–98.
- Mulligan, A.A.; Kuhnle, G.G.; Lentjes, M.A.; van Scheltinga, V.; Powell, N.A.; McTaggart, A.; Bhaniani, A.; Khaw, K.T. Intakes and sources of isoflavones, lignans, enterolignans, coumestrol and soya-containing foods in the Norfolk arm of the European Prospective Investigation into Cancer and Nutrition (EPIC-Norfolk), from 7 d food diaries, using a newly updated database. Public Health Nutr. 2013, 16, 1454–1462.
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Kuhnle, G.G.; Mulligan, A.A.; Touillaud, M.; Slimani, N.; Romieu, I.; Powell, N.; Tumino, R.; et al. Dietary intakes and food sources of phytoestrogens in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24-hour dietary recall cohort. Eur. J. Clin. Nutr. 2012, 66, 932–941.
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian adult soy protein and isoflavone intakes. Nutr. Cancer 2006, 55, 1–12.
- Jayachandran, M.; Xu, B. An insight into the health benefits of fermented soy products. Food Chem. 2019, 271, 362–371.
- Zhou, J.; Yuan, W.J. Effects of soy protein containing isoflavones in patients with chronic kidney disease: A systematic review and meta-analysis. Clin. Nutr. 2015, 35, 117–124.
- Wei, P.; Liu, M.; Chen, Y.; Chen, D.D. Systematic review of soy isoflavone supplements on osteoporosis in women. Asian Pac. J. Trop. Med. 2012, 5, 243–248.
- Harland, J.I.; Haffner, T.A. Systematic review, meta-analysis and regression of randomised controlled trials reporting an association between an intake of circa 25 g soya protein per day and blood cholesterol. Atherosclerosis 2008, 200, 13–27.
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food). Scientific opinion on the risk assessment for peri- and post-menopausal women taking food supplements containing isolated isoflavones. EFSA J. 2015, 13, 4246.
- Hazim, S.; Curtis, P.J.; Schär, M.Y.; Ostertag, L.M.; Kay, C.D.; Minihane, A.M.; Cassidy, A. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 694–702.
- Acharjee, S.; Zhou, J.R.; Elajami, T.K.; Welty, F.K. Effects of soy nuts and equol status on blood pressure, lipids and inflammation in postmenopausal women stratified by metabolic syndrome status. Metabolism 2015, 64, 236–243.
- Wong, J.M.; Kendall, C.W.; Marchie, A.; Liu, Z.; Vidgen, E.; Holmes, C.; Jackson, C.J.; Josse, R.G.; Pencharz, P.B.; Rao, A.V.; et al. Equol status and blood lipid profile in hyperlipidemia after consumption of diets containing soy foods. Am. J. Clin. Nutr. 2012, 95, 564–571.
- Thorp, A.A.; Howe, P.R.; Mori, T.A.; Coates, A.M.; Buckley, J.D.; Hodgson, J.; Mansour, J.; Meyer, B.J. Soy food consumption does not lower LDL cholesterol in either equol or nonequol producers. Am. J. Clin. Nutr. 2008, 88, 298–304.
- Jou, H.J.; Wu, S.C.; Chang, F.W.; Ling, P.Y.; Chu, K.S.; Wu, W.H. Effect of intestinal production of equol on menopausal symptoms in women treated with soy isoflavones. Int. J. Gynecol. Obstet. 2008, 102, 44–49.
- Akhlaghi, M.; Zare, M.; Nouripour, F. Effect of soy and soy isoflavones on obesity-related anthropometric measures: A systematic review and meta-analysis of randomized controlled clinical trials. Adv. Nutr. 2017, 8, 705–717.
- Fang, K.; Dong, H.; Wang, D.; Gong, J.; Huang, W.; Lu, F. Soy isoflavones and glucose metabolism in menopausal women: A systematic review and meta-analysis of randomized controlled trials. Mol. Nutr. Food Res. 2016, 60, 1602–1614.
- Cheng, P.F.; Chen, J.J.; Zhou, X.Y.; Ren, Y.F.; Huang, W.; Zhou, J.J.; Xie, P. Do soy isoflavones improve cognitive function in postmenopausal women? A meta-analysis. Menopause 2015, 22, 198–206.
- Van Die, M.D.; Bone, K.M.; Williams, S.G.; Pirotta, M.V. Soy and soy isoflavones in prostate cancer: A systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014, 113, E119–E130.
- Lethavy, A.; Marjoribanks, J.; Kronenberg, F.; Roberts, H.; Eden, J.; Brown, J. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst. Rev. 2013, 12.
- Taku, K.; Melby, M.K.; Kurzer, M.S.; Mizuno, S.; Watanabe, S.; Ishimi, Y. Effects of soy isoflavone supplements on bone turnover markers in menopausal women: Systematic review and meta-analysis of randomized controlled trials. Bone 2010, 47, 413–423.
- EFSA DNS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food). Scientific Opinion on the substantiation of health claims related to soy isoflavones and maintenance of bone mineral density (ID 1655) and reduction of vasomotor symptoms associated with menopause (ID 1654, 1704, 2140, 3093, 3154, 3590) (further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2847.
- Iwasaki, M.; Hamada, G.S.; Nishimoto, I.N.; Netto, M.M.; Motola, J., Jr.; Laginha, F.M.; Kasuga, Y.; Yokoyama, S.; Onuma, H.; Nishimura, H.; et al. Dietary isoflavone intake, polymorphisms in the CYP17, CYP19, 17beta-HSD1, and SHBG genes, and risk of breast cancer in case-control studies in Japanese, Japanese Brazilians, and non-Japanese Brazilians. Nutr. Cancer 2010, 62, 466–475.
- Low, Y.L.; Dunning, A.M.; Dowsett, M.; Luben, R.N.; Khaw, K.T.; Wareham, N.J.; Bingham, S.A. Implications of gene-environment interaction in studies of gene variants in breast cancer: An example of dietary isoflavones and the D356N polymorphism in the sex hormone-binding globulin gene. Cancer Res. 2006, 66, 8980–8983.
- Guadamuro, L.; Delgado, S.; Redruello, B.; Flórez, A.B.; Suárez, A.; Martínez-Camblor, P.; Mayo, B. Equol status and changes in faecal microbiota in menopausal women receiving long-term treatment for menopause symptoms with a soy-isoflavone concentrate. Front. Microbiol. 2015, 6, 777.
- Nakatsu, C.H.; Armstrong, A.; Cavijo, A.P.; Martin, B.R.; Barnes, S.; Weaver, C.M. Fecal bacterial community changes associated with isoflavone metabolites in postmenopausal women after soy bar consumption. PLoS ONE 2014, 9, e108924.
- Peeters, P.H.M.; Slimani, N.; van der Schouw, Y.T.; Grace, P.B.; Navarro, C.; Tjonneland, A.; Olsen, A.; Clavel-Chapelon, F.; Touillaud, M.; Boutron-Ruault, M.C.; et al. Variations in plasma phytoestrogen concentrations in European adults. J. Nutr. 2007, 137, 1294–1300.
- Ahuja, V.; Miura, K.; Vishnu, A.; Fujiyoshi, A.; Evans, R.; Zaid, M.; Miyagawa, N.; Hisamatsu, T.; Kadota, A.; Okamura, T.; et al. Significant inverse association of equol-producer status with coronary artery calcification but not dietary isoflavones in healthy Japanese men. Br. J. Nutr. 2017, 117, 260–266.
- Wu, J.; Oka, J.; Ezaki, J.; Ohtomo, T.; Ueno, T.; Uchiyama, S.; Toda, T.; Uehara, M.; Ishimi, Y. Possible role of equol status in the effects of isoflavone on bone and fat mass in postmenopausal Japanese women: A double-blind, randomized, controlled trial. Menopause 2007, 14, 866–874.
- Hall, M.C.; O’Brien, B.; McCormack, T. Equol producer status, salivary estradiol profile and urinary excretion of isoflavones in Irish Caucasian women, following ingestion of soymilk. Steroids 2007, 72, 64–70.
- Newton, K.M.; Reed, S.D.; Uchiyama, S.; Qu, C.; Ueno, T.; Iwashita, S.; Gunderson, G.; Fuller, S.; Lampe, J.W. A cross-sectional study of equol producer status and self-reported vasomotor symptoms. Menopause 2015, 22, 489–495.
- Nettleton, J.A.; Greany, K.A.; Thomas, W.; Wangen, K.E.; Adlercreutz, H.; Kurzer, M.S. The effect of soy consumption on the urinary 2:16-hydroxyestrone ratio in postmenopausal women depends on equol production status but is not influenced by probiotic consumption. J. Nutr. 2005, 135, 603–608.
- Hidayat, A. Effect of soy isoflavone supplementation on endothelial dysfunction and oxidative stress in equol-producing postmenopausal women. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 71–79.
- Van der Velpen, V.; Geelen, A.; Hollman, P.C.; Schouten, E.G.; van’t Veer, P.; Afman, L.A. Isoflavone supplement composition and equol producer status affect gene expression in adipose tissue: A double-blind, randomized, placebo-controlled crossover trial in postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 1269–1277.
- Crawford, S.L.; Jackson, E.A.; Churchill, L.; Lampe, J.W.; Leung, K.; Ockene, J.K. Impact of dose, frequency of administration, and equol production on efficacy of isoflavones for menopausal hot flashes: A pilot randomized trial. Menopause 2013, 20, 936–945.
- Pawlowski, J.W.; Martin, B.R.; McCabe, G.P.; McCabe, L.; Jackson, G.S.; Peacock, M.; Barnes, S.; Weaver, C.M. Impact of equol-producing capacity and soy-isoflavone profiles of supplements on bone calcium retention in postmenopausal women: A randomized crossover trial. Am. J. Clin. Nutr. 2015, 102, 695–703.
- Thomas, P.; Dong, J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006, 102, 175–179.
- Sosvorová, L.; Mikšátková, P.; Bičíková, M.; Kaňová, N.; Lapčík, O. The presence of monoiodinated derivates of daidzein and genistein in human urine and its effect on thyroid gland function. Food Chem. Toxicol. 2012, 50, 2774–2779.
- De la Parra, C.; Otero-Franqui, E.; Martinez-Montemayor, M.; Dharmawardhane, S. The soy isoflavone equol may increase cancer malignancy via up-regulation of eukaryotic protein synthesis initiation factor eIF4G. J. Biol. Chem. 2012, 287, 41640–41650.
- Gardana, C.; Simonetti, P. Long-term kinetics of daidzein and its main metabolites in human equol-producers after soymilk intake: Identification of equol-conjugates by UPLC-orbitrap-MS and influence of the number of transforming bacteria on plasma kinetics. Int. J. Food Sci. Nutr. 2017, 68, 496–506.
- Chadha, R.; Bhalla, Y.; Jain, A.; Chadha, K.; Karan, M. Dietary soy isoflavone: A mechanistic insight. Nat. Prod. Commun. 2017, 12, 627–634.
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29.
- Lehmann, L.; Esch, H.L.; Wagner, J.; Rohnstock, L.; Metzler, M. Estrogenic and genotoxic potential of equol and two hydroxylated metabolites of daidzein in cultured human Ishikawa cells. Toxicol. Lett. 2005, 158, 72–86.
- Hertrampf, T.; Schmidt, S.; Laudenbach-Leschowsky, U.; Seibel, J.; Diel, P. Tissue-specific modulation of cyclooxygenase-2 (Cox-2) expression in the uterus and the v. cava by estrogens and phytoestrogens. Mol. Cell. Endocrinol. 2005, 243, 51–57.
- Yang, Z.; Zhao, Y.; Yao, Y.; Li, J.; Wang, W.; Wu, X. Equol induces mitochondria-dependent apoptosis in human gastric cancer cells via the sustained activation of ERK1/2 pathway. Mol. Cells 2016, 39, 742–749.
- Shinkaruk, S.; Durand, M.; Lamothe, V.; Carpaye, A.; Martinet, A.; Chantre, P.; Vergne, S.; Nogues, X.; Moore, N.; Bennetau-Pelissero, C. Bioavailability of glycitein relatively to other soy isoflavones in healthy young Caucasian men. Food Chem. 2013, 135, 1104–1111.
- Rietjens, I.M.; Sotoca, A.M.; Vervoort, J.; Louisse, J. Mechanisms underlying the dualistic mode of action of major soy isoflavones in relation to cell proliferation and cancer risks. Mol. Nutr. Food Res. 2013, 57, 100–113.
- Joy, S.; Siow, R.C.; Rowlands, D.J.; Becker, M.; Wyatt, A.W.; Aaronson, P.I.; Coen, C.W.; Kallo, I.; Jacob, R.; Mann, G.E. The isoflavone equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cells. J. Biol. Chem. 2006, 281, 27335–27345.
- Rowlands, D.J.; Chapple, S.; Siow, R.C.; Mann, G.E. Equol-stimulated mitochondrial reactive oxygen species activate endothelial nitric oxide synthase and redox signaling in endothelial cells: Roles for F-actin and GPR30. Hypertension 2011, 57, 833–840.
- Kamiyama, M.; Kishimoto, Y.; Tani, M.; Utsunomiya, K.; Kondo, K. Effects of equol on oxidized low-density lipoprotein-induced apoptosis in endothelial cells. J. Atheroscler. Thromb. 2009, 16, 239–249.
- Gou, Z.; Jiang, S.; Zheng, C.; Tian, Z.; Lin, X. Equol inhibits LPS-induced oxidative stress and enhances the immune response in chicken HD11 macrophages. Cell. Physiol. Biochem. 2015, 36, 611–621.
- Zhang, T.; Hu, Q.; Shi, L.; Qin, L.; Zhang, Q.; Mi, M. Equol attenuates atherosclerosis in apolipoprotein E-deficient mice by inhibiting endoplasmic reticulum stress via activation of Nrf2 in endothelial cells. PLoS ONE 2016, 11, e0167020.
- Casagrande, F.; Darbon, J.M. Effect of structurally related flavonoids on cell cycle progression of human melanoma cells: Regulation of cyclin-dependent kinases CDK11 and CDK2. Biochem. Pharmacol. 2001, 61, 1205–1215.
- Mizushina, Y.; Shiomi, K.; Kuriyana, I.; Takahashi, Y.; Yoshida, H. Inhibitory effect of a major soy isoflavone, genistein, on human DNA topoisomerase II activity and cancer cell proliferation. Int. J. Oncol. 2013, 43, 1117–1124.
- Ono, M.; Ejima, K.; Higuchi, T.; Takeshima, M.; Wakimoto, R.; Nakano, S. Equol enhances apoptosis-inducing activity of genistein by increasing Bax/Bcl-xL expression ratio in MCF-7 human breast cancer cells. Nutr. Cancer 2017, 69, 1300–1307.
- Kim, E.Y.; Shin, J.Y.; Park, Y.J.; Kim, A.K. Equol induces mitochondria-mediated apoptosis of human cervical cancer cells. Anticancer Res. 2014, 34, 4985–4992.
- Itsumi, M.; Shiota, M.; Takeuchi, A.; Kashiwagi, E.; Inokuchi, J.; Tatsugami, K.; Kajioka, S.; Uchiumi, T.; Naito, S.; Eto, M.; et al. Equol inhibits prostate cancer growth through degradation of androgen receptor by S-phase kinase-associated protein 2. Cancer Sci. 2016, 107, 1022–1028.
- Lu, Z.; Zhou, R.; Kong, Y.; Wang, J.; Xia, W.; Guo, J.; Liu, J.; Sun, H.; Liu, K.; Yang, J.; et al. S-equol, a secondary metabolite of natural anticancer isoflavone daidzein, inhibits prostate cancer growth in vitro and in vivo, though activating the Akt/FOXO3a pathway. Curr. Cancer Drug Targets 2016, 16, 455–465.
- Yang, Z.P.; Zhao, Y.; Huang, F.; Chen, J.; Yao, Y.H.; Li, J.; Wu, X.N. Equol inhibits proliferation of human gastric carcinoma cells via modulating Akt pathway. World J. Gastroenterol. 2015, 21, 10385–10399.
- Harada, K.; Sada, S.; Sakaguchi, H.; Takizawa, M.; Ishida, R.; Tsuboi, T. Bacterial metabolite S-equol modulates glucagon-like peptide-1 secretion from enteroendocrine L cell line GLUTag cells via actin polymerization. Biochem. Biophys. Res. Commun. 2018, 501, 1009–1015.
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403.
- Horiuchi, H.; Usami, A.; Shirai, R.L.; Harada, N.; Ikushiro, S.; Sakaki, T.; Nakano, Y.; Inui, H.; Yamaji, R. S-Equol activates cAMP signaling at the plasma membrane of INS-1 pancreatic β-cells and protects against streptozotocin-induced hyperglycemia by increasing β-cell function in male mice. J. Nutr. 2017, 147, 1631–1639.
- Gopaul, R.; Knaggs, H.E.; Lephart, E.D. Biochemical investigation and gene analysis of equol: A plant and soy-derived isoflavonoid with antiaging and antioxidant properties with potential human skin applications. Biofactors 2012, 38, 44–52.
This entry is offline, you can click here to edit this entry!
