Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
5-(Hydroxymethyl)-2-furfural (HMF), represents a wide class of heterocycles and is formed as an intermediary product of the Maillard reaction or formed by carbohydrate dehydration in an acid medium. HMF also can be generated in significantly amounts at low temperatures during long periods of storage. The formation of HMF is affected by the concentration and type of sugar, acid, minerals, pH as well as amino acids.
- toxicology
- heat-borne
- Maillard reaction
- 5-hydroxymethylfurfural
- toxicant
1. Introduction
During food processing (cooking/heat treatment), several substances are formed. Some of these substances may add taste, color and texture while minimizing harmful germs. Some newly-formed compounds may have antimicrobial, antiallergenic and antioxidant activity [1]. Besides these beneficial substances, some other substances should be cautiously evaluated.
5-(Hydroxymethyl)-2-furfural (HMF), a heat-induced food toxicant, is formed in many food items, and causes several adverse effects in animals. HMF has been found in a wide variety of food products like dried fruit, fruit juice, caramel products, coffee, bakery products, malt and vinegar [2,3]. It has been also detected in cigarette smoke and chewing tobacco [4,5]. The toxicological effects of HMF have been demonstrated in several experimental animals and in cultured mammalian cells [6,7,8,9,10]. The daily human intake of HMF was reviewed by Husøy et al. [11] and ranged from 30 and 150 mg/person/day, a dose much higher than that of other food heat-borne toxicants like furan and acrylamide [12,13]. An estimation by the Federal Institute of Risk Assessment [14] showed a range from 4 to 30 mg HMF/person/day.
Cytotoxic, genotoxic, mutagenic and carcinogenic activities of HMF in rats and mice have been reported earlier [9,15]. Lee et al. [16] reported that HMF is an indirect bacterial mutagen due to its active metabolite, sulfuric acid ester 5-sulfo-oxymethylfurfural (SMF). The metabolic formation of SMF was illustrated by activated mutagenicity of HMF in the presence of rat hepatic cytosol enriched with the sulfogroup donor, 3′-phosphoadenosine-5′-phosphosulfate (PAPS). SMF was found to act as a mutagen in mammalian cultured cells and to initiate tumours in mouse skin [17]. Sulfur conjugation has been found to play a central role in metabolic activation, mutagenicity, and carcinogenicity of several environmental toxicants that include aromatic amines, alkenylbenzenes, and polynuclear aromatic hydrocarbons [6], indicating that the toxic effects of HMF can be metabolically enhanced via some of its metabolites.
2. Occurrence and Dietary Exposure to HMF
The level of measurable HMF in food materials is clearly associated with the heat of reaction. The formation of HMF is an essential marker of temperature changes during storage in different food products such as juice and honey [22]. HMF can be formed in food products via different routes such as acid catalysed degradation of reducing sugars or through Maillard reactions. The formation of HMF is not only an indicator of the storage conditions and food quality, but also indicates the potential of occurrence of contamination during Maillard reactions, or by dehydration [23]. In addition to the presence of HMF in honey (Figure 1) and in preserved fruits (> 1 g/kg), it also occurs in other food ingredients including instant coffee or caramel (more than 6.2 g/kg), milk, citrus juices, apple juice, baked foods, breakfast cereal, and tomato byproducts. HMF is originated from sugars during food processing. Humans consume many different kinds of food products which are usually subjected to thermal manipulation steps such as boiling, pasteurization, roasting or baking before their consumption,. Throughout the heat course or when preparing food for storage, the Maillard reaction could occur and HMF be formed according to either the processing or storage conditions [24].
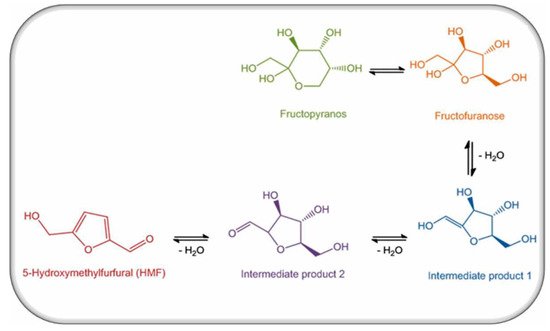
Figure 1. Production of HMF in honey.
Polovková and Šimko [23] detected HMF levels by high performance liquid chromatography supported with a diode array detector (HPLC-DAD) at 284 nm, and their findings showed the presence of HMF in 25 kinds of brown sugar, 15 kinds of which had HMF values that ranged from 0.17 to 6.45 mg/kg, while 13 kinds of white sugar had zero HMF. The authors attributed the occurrence of HMF in brown sugar to a lack of refining or as a result of adding treacle during the production process. These results were compatible with a previous study, where values of HMF in sugar-containing products were estimated and recorded (10.9–16.4, and 12.3–23.3 mg/kg) for light- and dark-brown sugars, respectively. The mechanism of HMF formation from simple sugars is illustrated in Figure 2.
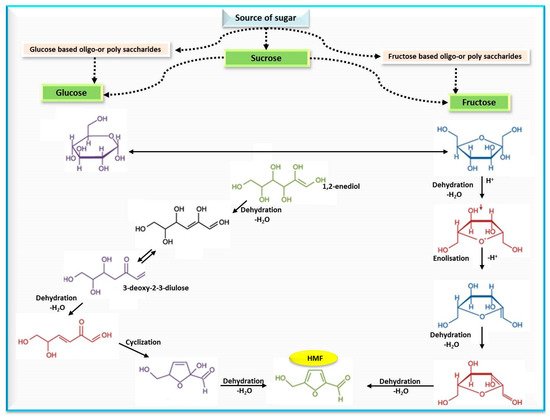
Figure 2. Mechanism of HMF formation from simple sugars.
Mańkowska et al. [25] reported that distinct HMF levels could be found in 41 kinds of food. Wheat bread with cranberries displayed the highest HMF level (210 mg/kg), breakfast cereals, (i.e., honey wheat loops; 85.09 mg/kg), while depleted values of HMF were found in gluten-free sponge cakes or whole-grain oatmeal. Sweetened breakfast cereals included 25.55 mg/kg HMF, 39% higher than the average content in bakery food products (18.40 mg/kg). Moreover, the HMF average values in cereals, (i.e., combined grains (240 mg/kg), cornflakes (7–114 mg/kg) and wheat-associated cereals (6–132 mg/kg) were also high [26,27]. Food products that undergo fermentation and some types of flour are usually used in bread and other bakery products. HMF values in bread manufactured from rye flour was recorded to be the highest level (average 26.88 mg/kg) and could be attributed to the high amino acid composition.
Also, some kinds of preserved fruits such as apple, strawberries, raisins, palms, cranberries, and red currants normally have great levels of HMF. HMF contents in rice-wheat flakes were determined to range from 6.78 to 11.70 mg/kg, while a lower level of HMF (6.06 mg/kg) was detected in by-products including raisins or plums. However, red fruits such as apples, red currants and strawberries have the highest levels of HMF [25]. Hence, white bread with preserved fruits has higher HMF levels than white bread alone [28,29]. Coffee, a common drink, also contains HMF and its concentration depends on the kind of coffee (plunger-brewed coffees, filtered coffee, mocha or espresso) and amount of sugar added. Mortas et al. [30] determined HMF in Turkish coffees (either instant brand or traditionally prepared) by HPLC supported with a diode array indicator. They reported that prior to steeping, instant and traditional Turkish coffee specimens contain HMF levels of 213.02–238.99 and 336.03–362.05 mg/kg, respectively. After preparation, the HMF content was elevated in instant coffee by 32.29–55.83%, while the concentration of HMF in traditional coffee increased by 74.12–224.75%.
Arribas-Lorenzo and Morales [31] used reversed-phase chromatography supported by UV-detection to estimate HMF concentrations in three kinds of ground coffee consumed by the Spanish population and observed clear variations in the contents. HMF values were 110 mg/kg (original coffee: manufactured by ordinary roasting of coffee beans), 625 mg/kg (torrefacto coffee: manufactured by mixing sucrose before the roasting step) and 1734 mg/kg (blended coffee: a mixture of original and ground torrefacto coffee in different ratios), while soluble coffee had the highest value (2480 mg/kg). The authors showed that the HMF consumption along with heavy coffee adult consumers in Spain was about 122.42 μg/kg/day, and suggesting an important thermal contribution to HMF generation.
HMF can be formed in dairy products by side chemical reactions during thermal sterilization and browning processes [32]. Indeed, the Maillard reaction is undesirable in infant milk because these the precursors might be the only source of lysine for babies [33]. Identical HPLC conditions were used to measure free and total HMF in liquid and powdered infant milk formulas to estimate the effect of the length and temperature of storage on the extent of the Maillard reaction in infant milk. A linear increase in the concentration of free and total HMF was found with increasing storage time and temperature in both powdered and liquid infant milk, however, values of total and free HMF were higher in powdered infant milk in comparison with the liquid milk of the same commercial brand. These differences may due to the temperature treatments applied to powdered milk during the manufacturing process [34]. There were no significant differences in total HMF content between milk stored at 4 °C or those stored at 8 °C, while storage this milk for 24 weeks caused a significant increase in the total HMF content by about 1.7 fold compared to the value estimated directly after production. However, storing the milk for the same period (24 weeks) at room temperature showed a 2.06-fold increase in HMF content [35]. These findings also indicate that both storage temperature and storage time can increase the HMF content in the UHT sterilised milk.
3. Acceptable Daily Intake of HMF
The medium and high daily HMF consumption has been estimated at 5.26 mg and 8.57 mg respectively [41]. Most of this dietary exposure comes from coffee, which accounts for about 50% of the estimated total HMF consumption in Spain [42] and about 63% in Norway [11]. The estimated level of HMF exposure ranged from 30 to 150 mg per person [43]. Findings from earlier studies reported that the toxic effects of HMF were observed with a dose more than 75 mg/kg body weight [44]. Zaitsev et al. [45] reported 2 mg of HMF/kg body weight is suggested as an acceptable daily intake from food for human beings, while findings from other studies observed that daily consumption of HMF should range from 2–30 mg per person/day [41,46].
4. Uses of HMF
HMF can be widely used as an indicator of the quality of food products such as coffee [47], milk [32], honey [48] or processed fruits [2]. HMF was also used for checking the thermal procedures applied to commercial cereal products such as breakfast cereals [49], pasta preservation [50], bread slice toasting [51] or bread baking [28], as well as baby cereals [52]. Thermal processing of food plays a key role in improving the digestibility and absorption as well as the availability of bioactive compounds due to cell breakdown. However, aggressive and long high heat processing may lead to damage and loss of some bioactive compounds. It has been shown that HMF can be used as a useful marker to control heat processing time and the type and intensity of heat-treating of cooked vegetables. In addition, an association has been found between HMF and the antioxidant capacity potential of vegetables exposed to various cooking techniques [53].
5. Metabolism of HMF
It was demonstrated from oral gavage administration trials of [14C]-HMF at different dosages (0.08–500 mg/kg body weight) that HMF extensively passed through the digestive canal in both rats and mice [54]. In a Caco2 cell line, Delgado-Andrade et al. [55] reported that HMF absorption could be increased when cells were subjected to a higher HMF level. It has been demonstrated that HMF absorption can be affected also by food composition such as fiber content. Moreover, the bioavailability of HMF in three commercial breakfast cereals ranged from 4.98 to 12.99%. This variation may due to variations in the composition of each breakfast cereals, while fiber content also plays an essential role [55].
The main biotransformation pathway of HMF occurred through HMF oxidation to 5-hydroxymethyl-2-furanoic acid (HMFA) followed by glycine conjugation to form N-(5-hydroxymethyl-2-furoyl)glycine (HMFG) as the principal metabolite eliminated in the urine [41]. In rat and human trials, the HMFA/HMFG ratio dropped as the HMF dose increased, suggesting that confined glycine could minimize the conjugation reaction rate, leading to the elimination of either free furoic acid (FA) or 2,5-furandicarboxylic acid (FDCA) via other routes [56].
On the other hand, a small human study was conducted on seven adult persons, where the urine excretion of HMF was determined to estimate the residual HMF in the body. The participants consumed 20 g of plum jam contain 24 mg of HMF, and only 163 µg were detected in urine after 6 h, indicating that 99.25% of the consumed HMF remained in the body [3].
In addition, HMF has been also observed to be transformed in vivo into 5-sulfoxymethyfurfural (SMF) via sulphonation of its allylic hydroxyl active group initiated by sulfotransferases (SULTs) and the sulpho group donor 3-phosphoadenosine-5--phosphosulphate (PAPS). SMF is a unstable form, however, it has been detected in the bloodstream of HMF-treated mice and humans, indicating that HMF was transformed into SMF in an vivo model [7,46].
Some trials were conducted using [14C]-HMF. The results indicated that dietary HMF was obviously and clearly transformed and eliminated via urine or to a lower degree in hepatic tissue [54].
6. Toxicity of HMF
Several studies have reported various adverse effects of HMF on human health as described in the following sections and summarized in Figure 3.
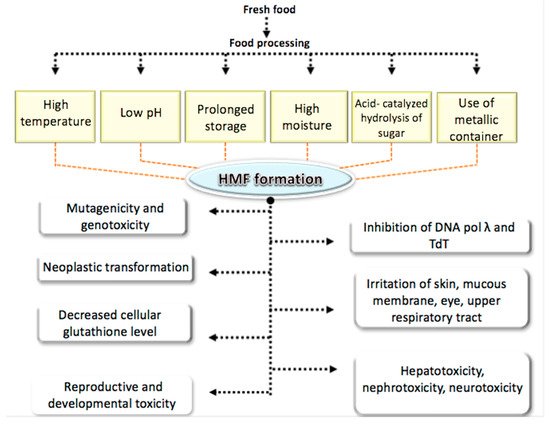
Figure 3. Adverse effect of HMF on human health.
7. Prooxidant Effects of HMF
Janzowski et al. [43] found that a high concentration of HMF (120 mM) caused a depletion in the concentration of glutathione (GSH) in different mammalian cultured cells. However, there was no detectable damage to DNA. Furfural is oxidized in liver into pyromucic acid which has a toxic effect on hepatocytes, however, creation of glycine in the liver helps in furfural detoxification via conjugate with pyromucic acid and excretion in urine [70,71]. However, there is a shortage of studies related to the in vivo prooxidant effects of HMF and the probability of being a mechanism of its toxic effect.
8. Carcinogenic Effects of HMF
It has been demonstrated that consumption of HMF in a high dose may result in the initiation of tumorigenic activities. HMF and its derivative SMF induces neoplastic transformations in different tissues. Findings from a previous study reported that HMF has the potential to induce aberrant crypt foci (ACF) in a dose-dependent manner [9,72]. However, HMF is a weak intestinal carcinogen. Moreover, some HMF derivatives such as sulfoxymethyl and chloromethyl were found to exhibit high skin tumor-initiating activity [65]. When topically applied to the skin of B6C3F1 mice, HMF showed lower induction of skin papilloma than its derivative SMF [65]. In contrast, Florian et al. reported that neither HMF nor its metabolite SMF caused ACF and intestinal carcinoma [73]. Peroral application of 188 mg HMF/kg for 104 weeks could induce hepatocellular adenomas in female B6C3F1 mice in comparison to control mice [59]. The dosage of 188 mg HMF/kg clearly elevated the prevalence of liver adenoma in female mice over a 24 month trial [59].
This entry is adapted from the peer-reviewed paper 10.3390/molecules25081941
This entry is offline, you can click here to edit this entry!
