Vasopressor therapy is to restore organ perfusion so as to limit the risk of multiple organ failure and death.
- vasopressor
- shock
- norepinephrine
1. Introduction
Because fluid and vasopressors are the main treatments for shock, they are used on a day-to-day basis as symptomatic treatment for arterial hypotension. Vasoplegia is associated with vasodilation and vascular hypo-responsiveness, and involves multiple mechanisms [1]. Despite the emergence of new vasopressor agents, norepinephrine is still the recommended first-line agent [2]. One problem with the use of vasopressors is the risk of side effects and the ensuing need for intensive care management, which is costly.Studies have demonstrated that vasopressor use can be associated with specific side effects, and prolonged use may be associated with mortality [3][4].A study has demonstrated that implementing vasopressor sparing strategies is associated with lower morbidity and ICU (intensive care unit) length of stays [4]. Numerous reviews have investigated the different types of vasopressors and their hemodynamic effects [1][5][6][7][8], but, to date, no review has specifically focused on therapeutic and non-therapeutic strategies associated with the sparing effect for vasopressor use.Thus, we do not have meta-analyses or reviews evaluating the vasopressor-sparing strategies.
2. Pharmacological Strategies
3. Hemodynamic Strategies
4. Proposed Algorithm
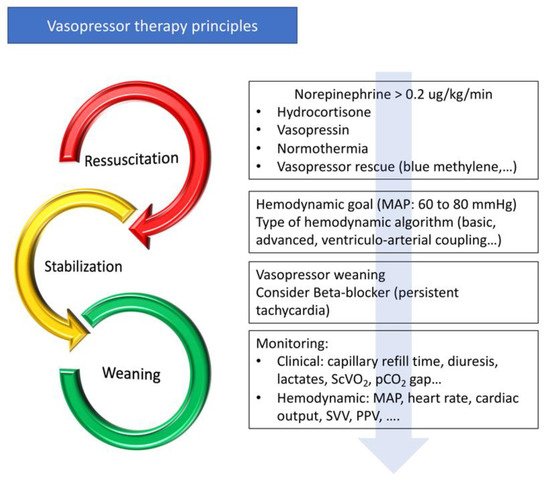
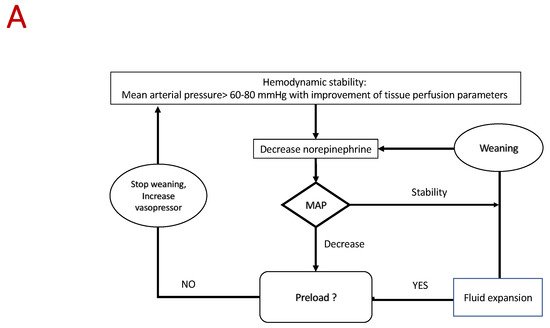
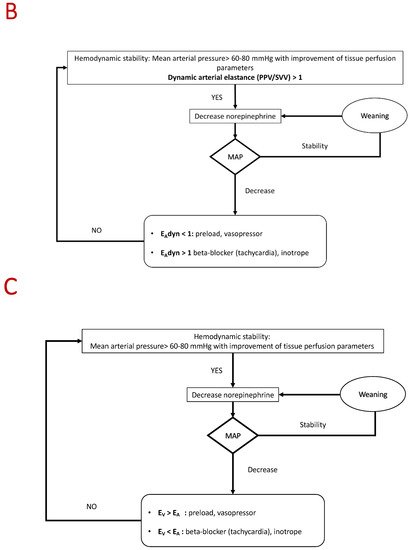
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/jcm10143164
References
- Landry, D.W.; Oliver, J.A. The Pathogenesis of Vasodilatory Shock. N. Engl. J. Med. 2001, 345, 588–595.
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377.
- Lamontagne, F.; Day, A.G.; Meade, M.O.; Cook, D.J.; Guyatt, G.H.; Hylands, M.; Radermacher, P.; Chrétien, J.M.; Beaudoin, N.; Hébert, P.; et al. Pooled analysis of higher versus lower blood pressure targets for vasopressor therapy septic and vasodilatory shock. Intensive Care Med. 2018, 44, 12–21.
- Guinot, P.-G.; Abou-Arab, O.; Guilbart, M.; Bar, S.; Zogheib, E.; Daher, M.; Besserve, P.; Nader, J.; Caus, T.; Kamel, S.; et al. Monitoring dynamic arterial elastance as a means of decreasing the duration of norepinephrine treatment in vasoplegic syndrome following cardiac surgery: A prospective, randomized trial. Intensive Care Med. 2017, 43, 643–651.
- Hollenberg, S.M. Concise Clinical Review Vasoactive Drugs in Circulatory Shock. Am. J. Respir. Crit. Care Med. 2011, 183, 847–855.
- Bangash, M.N.; Kong, M.L.; Pearse, R.M. Use of inotropes and vasopressor agents in critically ill patients. Br. J. Pharmacol. 2012, 165, 2015–2033.
- Russell, J.A. Vasopressor therapy in critically ill patients with shock. Intensive Care Med. 2019, 45, 1503–1517.
- Russell, J.A.; Gordon, A.C.; Williams, M.D.; Boyd, J.H.; Walley, K.R.; Kissoon, N. Vasopressor Therapy in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2021, 42, 59–77.
- Landry, D.W.; Levin, H.R.; Gallant, E.M.; Ashton, R.C.; Seo, S.; D’Alessandro, D.; Oz, M.C.; Oliver, J.A. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation 1997, 95, 1122–1125.
- Jeon, K.; Song, J.-U.; Chung, C.R.; Yang, J.H.; Suh, G.Y. Incidence of hypotension according to the discontinuation order of vasopressors in the management of septic shock: A prospective randomized trial (DOVSS). Crit. Care 2018, 22, 131.
- Hammond, D.A.; Sacha, G.L.; Bissell, B.D.; Musallam, N.; Altshuler, D.; Flannery, A.H.; Lam, S.W.; Bauer, S.R. Effects of Norepinephrine and Vasopressin Discontinuation Order in the Recovery Phase of Septic Shock: A Systematic Review and Individual Patient Data Meta-Analysis. Pharmacotherapy 2019, 39, 544–552.
- Wu, Z.; Zhang, S.; Xu, J.; Xie, J.; Huang, L.; Huang, Y.; Yang, Y.; Qiu, H. Norepinephrine vs Vasopressin: Which Vasopressor Should Be Discontinued First in Septic Shock? A Meta-Analysis. Shock 2020, 53, 50–57.
- Bellissant, E. Effect of hydrocortisone on phenylephrine– mean arterial pressure dose-response relationship in septic shock. Clin. Pharmacol. Ther. 2000, 68, 293–303.
- Cooper, M.S.; Stewart, P.M. Corticosteroid Insufficiency in Acutely Ill Patients. N. Engl. J. Med. 2003, 348, 727–734.
- Annane, D. Corticosteroids for septic shock. In Critical Care Medicine; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001.
- Morelli, A.; Ertmer, C.; Westphal, M.; Rehberg, S.; Kampmeier, T.; Ligges, S.; Orecchioni, A.; D’Egidio, A.; D’Ippoliti, F.; Raffone, C.; et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: A randomized clinical trial. JAMA J. Am. Med. Assoc. 2013, 310, 1683–1691.
- Morelli, A.; Singer, M.; Ranieri, V.M.; D’Egidio, A.; Mascia, L.; Orecchioni, A.; Piscioneri, F.; Guarracino, F.; Greco, E.; Peruzzi, M.; et al. Heart rate reduction with esmolol is associated with improved arterial elastance in patients with septic shock: A prospective observational study. Intensive Care Med. 2016, 42, 1528–1534.
- Bertini, P.; Guarracino, F. Septic Shock and the Heart. Curr. Anesthesiol. Rep. 2019, 9, 165–173.
- Angus, D.C.; Barnato, A.E.; Bell, D.; Bellomo, R.; Chong, C.R.; Coats, T.J.; Davies, A.; Delaney, A.; Harrison, D.A.; Holdgate, A.; et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: The ARISE, ProCESS and ProMISe Investigators. Intensive Care Med. 2015, 41, 1549–1560.
- Hamzaoui, O.; Georger, J.F.; Monnet, X.; Ksouri, H.; Maizel, J.; Richard, C.; Teboul, J.L. Early administration of norepinephrine increases cardiac preload and cardiac output in septic patients with life-threatening hypotension. Crit. Care 2010, 14, 1–9.
- Monnet, X.; Jabot, J.; Maizel, J.; Richard, C.; Teboul, J.-L. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients*. Crit. Care Med. 2011, 39, 689–694.
- Malbrain, M.L.N.G.; Van Regenmortel, N.; Saugel, B.; De Tavernier, B.; Van Gaal, P.J.; Joannes-Boyau, O.; Teboul, J.L.; Rice, T.W.; Mythen, M.; Monnet, X. Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensive Care. 2018, 8, 1–16.
- Bar, S.; Nguyen, M.; Abou-Arab, O.; Dupont, H.; Bouhemad, B.; Guinot, P.-G. Dynamic Arterial Elastance Is Associated With the Vascular Waterfall in Patients Treated With Norepinephrine: An Observational Study. Front. Physiol. 2021, 12, 514.
- Merouani, M.; Guignard, B.; Vincent, F.; Borron, S.W.; Karoubi, P.; Fosse, J.-P.; Cohen, Y.; Clec’h, C.; Vicaut, E.; Marbeuf-Gueye, C.; et al. Norepinephrine weaning in septic shock patients by closed loop control based on fuzzy logic. Crit. Care 2008, 12, R155.
- Lamontagne, F.; Richards-Belle, A.; Thomas, K.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Camsooksai, J.; Darnell, R.; Gordon, A.C.; Henry, D.; et al. Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Older Critically Ill Patients with Vasodilatory Hypotension: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2020.
- Marshall, J.C. Choosing the Best Blood Pressure Target for Vasopressor Therapy. JAMA J. Am. Med. Assoc. 2020, 323, 931–933.
- Schmittinger, C.A.; Torgersen, C.; Luckner, G.; Schröder, D.C.H.; Lorenz, I.; Dünser, M.W. Adverse cardiac events during catecholamine vasopressor therapy: A prospective observational study. Intensive Care Med. 2012, 38, 950–958.
- Buckley, M.S.; Barletta, J.F.; Smithburger, P.L.; Radosevich, J.J.; Kane-Gill, S.L. Catecholamine Vasopressor Support Sparing Strategies in Vasodilatory Shock. Pharmacother. J. Hum. Pharmacol. Drug. Ther. 2019, 39, 382–398.
- Hajjar, L.A.; Vincent, J.L.; Barbosa Gomes Galas, F.R.; Rhodes, A.; Landoni, G.; Osawa, E.A.; Melo, R.R.; Sundin, M.R.; Grande, S.M.; Gaiotto, F.A.; et al. Vasopressin versus Norepinephrine in Patients with Vasoplegic Shock after Cardiac Surgery. Anesthesiology 2017, 126, 85–93.
- Nguyen, M.; Abou-Arab, O.; Bar, S.; Dupont, H.; Bouhemad, B.; Guinot, P.-G. Echocardiographic measure of dynamic arterial elastance predict pressure response during norepinephrine weaning: An observational study. Sci. Rep. 2021, 11, 1–7.
- Nguyen, M.; Berhoud, V.; Bartamian, L.; Martin, A.; Ellouze, O.; Bouhemad, B.; Guinot, P.-G. Agreement between different non-invasive methods of ventricular elastance assessment for the monitoring of ventricular–arterial coupling in intensive care. J. Clin. Monit. Comput. 2019, 34, 1–9.
- Guinot, P.-G.; Bernard, E.; Levrard, M.; Dupont, H.; Lorne, E. Dynamic arterial elastance predicts mean arterial pressure decrease associated with decreasing norepinephrine dosage in septic shock. Crit. Care 2015, 19, 14.
- Bar, S.; Leviel, F.; Abou Arab, O.; Badoux, L.; Mahjoub, Y.; Dupont, H.; Lorne, E.; Guinot, P.-G. Dynamic arterial elastance measured by uncalibrated pulse contour analysis predicts arterial-pressure response to a decrease in norepinephrine. Br. J. Anaesth. 2018, 7, 1–7.
- Guinot, P.-G.; Longrois, D.; Kamel, S.; Lorne, E.; Dupont, H. Ventriculo-Arterial Coupling Analysis Predicts the Hemodynamic Response to Norepinephrine in Hypotensive Postoperative Patients: A Prospective Observational Study. Crit. Care Med. 2018, 46, e17–e25.
- Kelly, R.P.; Ting, C.T.; Yang, T.M.; Liu, C.P.; Maughan, W.L.; Chang, M.S.; Kass, D.A. Effective arterial elastance as index of arterial vascular load in humans. Circulation 1992, 86, 513–521.
- Garcia, M.I.M.; Jian, Z.; Settels, J.J.; Hatib, F.; Cecconi, M.; Pinsky, M.R. Reliability of effective arterial elastance using peripheral arterial pressure as surrogate for left ventricular end-systolic pressure. J. Clin. Monit. Comput. 2019, 33, 803–813.
- Chang, M.C.; Mondy, J.S.; Meredith, J.W.; Miller, P.R.; Owings, J.T.; Holcroft, J.W. Clinical application of ventricular end-systolic elastance and the ventricular pressure-volume diagram. Shock 1997, 7, 413–419.
- Chen, C.-H.; Fetics, B.; Nevo, E.; Rochitte, C.E.; Chiou, K.-R.; Ding, P.-A.; Kawaguchi, M.; Kass, D.A. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J. Am. Coll. Cardiol. 2001, 38, 2028–2034.
- Kass, D.A.; Kelly, R.P. Ventriculo-arterial coupling: Concepts, assumptions, and applications. Ann. Biomed. Eng. 1992, 20, 41–62.
- Wijnberge, M.; Geerts, B.F.; Hol, L.; Lemmers, N.; Mulder, M.P.; Berge, P.; Schenk, J.; Terwindt, L.E.; Hollmann, M.W.; Vlaar, A.P.; et al. Effect of a Machine Learning–Derived Early Warning System for Intraoperative Hypotension vs Standard Care on Depth and Duration of Intraoperative Hypotension During Elective Noncardiac Surgery. JAMA 2020, 323, 1052–1060.
- Venkatesh, B.; Khanna, A.K.; Cohen, J. Less is more: Catecholamine-sparing strategies in septic shock. Intensive Care Med. 2019, 45, 1810–1812.
