Bone tissue regeneration in orthopedic and maxillofacial surgery remains a common challenge. Trauma, tumors, infectious diseases, biochemical disorders, congenital disorders or abnormal skeletal development are the cause of bone defects, resulting in functional, esthetic and psychological defects in patients. Natural healing of skeletal structure is relatively limited and requires assistance during pathological conditions such as severe injuries, osteoporosis, osteosarcoma and infection.
- MMP-cleavable peptides
- crosslinking
- hydrogels
- degradation
- bone regeneration
1. Introduction
Autogenous bone was identified as the gold standard for bone defects and retained perfect biocompatibility, but it could not fully satisfy the requirements due to low yield, iatrogenic injury and risk [1]. Other solutions such as allografts, xenografts and bone substitute materials hold corresponding shortcomings in terms of, for example, immune response, infectious risk and disease transmission [2][3]. Therefore, a further sustainable and high-yielding strategy is required, which leads us to tissue engineering methods. Numerous studies have recently introduced bioactive scaffolds and their interaction with adjacent bony tissues, and hydrogels have received attention due to their excellent biocompatibility, biodegradability and plasticity [4][5][6].
With their hydrophilic polymeric networks, hydrogels are considered the most promising polymer scaffold in bone tissue engineering [7], and the modification of their permeability and stiffness enables substance exchanges and cell function [8][9]. As the basis and guiding principle of bone regeneration, the degradation behavior of hydrogels is directly related to the speed and quality of bone repair [10]. Specifically, hydrogels in bone regeneration should be constructed by biocompatible materials and hold enough stability for cell activity at an early stage [11][12]. Along with cell growth and microstructural remodeling, biodegradation of hydrogels is required to create appropriate space for the incoming inhabitants. Despite the natural and synthetic polymers used in their preparation, the degradation solution of hydrogels mainly takes into account temperature, pH, light irradiation, ultrasound and enzymes, among other aspects. [13][14][15]. Among them, enzymatically responsive hydrogels are well-recognized at present for their controlled and tunable degradation adapted to in vivo circumstances [16][17].
Response and adaption under environmental variation are intrinsic properties of all biosystems, as well as biomaterials [18]. The transformation of spatial configurations, physical properties or structural stability under proper stimulation helps in the degradation of bone fillers and the release of bioactive cargoes. Enzymes were valued as a promising trigger for novel responsive polymers, considering their biological origin, efficiency and high selectivity [19]. Leading-edge research reported that clustered regularly interspaced short palindromic repeats (CRISPR)-associated enzymes could be utilized to cleave DNA cargoes in responsive hydrogels and for the delivery of genetic information [20]. Remarkably, enzyme levels vary with in vivo microenvironments and biological behaviors, and this variation was used in a novel strategy that integrates enzymatic reaction and controlled release [21]. For instance, a smart hydrogel constructed by glutathione-modified collagen and MMP-cleavable peptide targeted myocardial infarction and ameliorating myocardium remodeling in vivo in a “release on-demand” manner [22]. Particularly, it was revealed that MMPs are involved in bone remolding. Thus, the MMP-cleavable peptides-based hydrogels are promising candidates for bone tissue engineering.
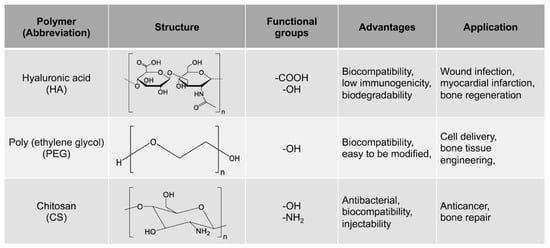
The growing demand for MMP-cleavable peptides-based hydrogel as a platform for biomedical applications exhibits a strong need for a timely review on a wide range of their fabrication and applications in bone repair. This review discusses the latest advances in MMP-cleavable peptides-based hydrogels for biomedical applications in bone regeneration. The MMP-cleavable peptides are introduced as crosslinkers for hydrogels. The three commonly used MMP-cleavable peptides-based hydrogels, including Poly(ethylene glycol) (PEG)-, hyaluronic acid (HA)- and chitosan (CS)-based hydrogels, are then highlighted. The advantages and limitations of using these hydrogels along with their different synthesis methods are summarized. Additionally, their most recent advances in the field of bone science, including hydrogel-based 3D in vitro models and bone healing, are subsequently reviewed. Finally, the current challenges and future perspectives of MMP-cleavable peptides-based hydrogels are briefly discussed.
2. General Materials for MMP-Cleavable Peptides-Based Hydrogels
Poly (ethylene glycol) (PEG) is a hydrophilic polymer that has the characteristics of biocompatibility and bioinertia, and it can support cell growth after the addition of the appropriate protease-sensitive connectors and cell adhesion sites [23]. Therefore, PEG hydrogel is a promising synthetic hydrogel. PEG hydrogels have interconnected microporous networks that provide continuous nutrient flow, cell growth and vascularization of engineering tissue ( Figure 1 ). Studies showed that PEG hydrogel helps to maintain the phenotype of natural heart valve cells [24], optimize cell viability and morphology [25], and promote the production of extracellular matrix [26]. Dai et al. prepared a kind of stromal cell-derived factor-1-α-loaded MMP degradable PEG hydrogel [27]. The experimental data show that the hydrogel has good biocompatibility, can promote the recruitment of mesenchymal stem cells, can promote the phenotypic polarization of M2 macrophages, and has good tissue remodeling ability. The hydrogel can also improve the adhesion, activity and proliferation of bone marrow mesenchymal stem cells (BMSCs) and promote the differentiation of BMSCs into valvular interstitial-like cells.
In addition, PEG hydrogels can be modified to meet the needs of specific applications in vitro and in vivo [5][28][29][30][31]. Metzger et al. cross-linked Streptavidin with PEG to prepare hydrogel, which can release immobilized growth factor (GF) and does not depend on the degradation of hydrogel [32]. Research data show that through the appropriate design of the release system, GF can be released by PEG hydrogels in a soluble form that is more effective than the supplementary cell culture medium for local delivery.
Moreover, PEG hydrogel is widely used in cell delivery and bone tissue engineering [33]. Sridhar et al. developed a peptide- and protein-functionalized PEG hydrogel. After being co-cultured with the hydrogel for 14 days, chondrocytes significantly increased the deposition of glycosaminoglycans and collagen, maintained a high level of activity, and produced a more widely distributed matrix. This shows that hydrogel can promote the production of cartilage matrix [34].
It was reported that PEG hydrogel can be used as a blank skeleton, in which multiple scaffolds with various functions can be systematically introduced into the scaffold to allow integrin binding [35], proteolysis and degradation [36][23], and even local isolation of growth factors [37]. Therefore, PEG hydrogels with specific material compositions can be used to guide mesenchymal stem cells to differentiate into specific types of chondrocytes [38]. Nguyen et al. designed and synthesized a three-layer composite hydrogel, based on PEG, that was doped with chondroitin sulfate, metalloproteinase-sensitive peptides and HA [39]. The results show that the hydrogel can not only induce MSCs to differentiate into chondrocytes, but also customize the phenotype and matrix production pattern of differentiated cells according to the specific region of articular cartilage by changing the material composition.
3. Synthesis of MMP-Cleavable Peptides-Based Hydrogels
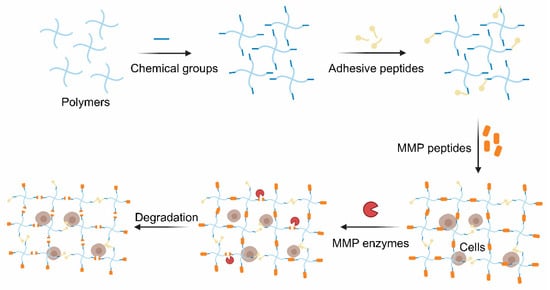
The thiol groups of cysteine usually act as a crosslinker in MMP-cleavable peptides. Although some MMP-cleavable peptides could be crosslinked with polymers by introducing chemical groups via the grafting of amino acids to peptides, tt is easier to introduce some functional groups into the polymers to construct hydrogels with the amino acid sequences. Several common methods of polymer modification are discussed below.
Norbornene (NB) groups, which are also molecule linkers, have attracted increasing attention because their photo-crosslink property and have been widely introduced into biomaterials for use as a bioink in bioprinting [40][41][42]. It is well-known that the photochemical reaction of the NB group holds a speedy reaction rate under physiological pH and temperature, and that the reactions could occur at relatively low radical concentrations [43]. These advantages demonstrate that introducing the NB group into biomaterials might be a promising solution in biomedicine and tissue engineering. Gelatin is a natural polymer, which exhibits cell-interactive properties, and could be easily modified due to its diverse chemical groups, including -OH, -COOH and -NH2. Therefore, gelatin could employ an NB group using 5-norbornene-2-carboxylic acid in the reaction of the carboxylic acid and the primary amines [43]. The norbornene derivant could also be utilized in NB group insertion; Guo et al. synthesized norbornene-collagen that was obtained from acidic collagen after reacting with carbic anhydride [44].
In addition, PEG, which is identified as one of the most common synthesis polymers, also combines with the NB group under the appropriate circumstances. Eight-arm PEG-hydroxyl, dissolved in dichloromethane (DCM) with pyridine and 4-Dimethylaminopyridine (DMAP), could introduce NB groups via an overnight reaction with 5-norbornene-2-carboxylic acid and N,N’-dicyclohexylcarbodiimide under nitrogen conditions [45]. The hydrogel could be formed with MMP-cleavable peptides under ultraviolet light (UV) with lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) and elevated alkaline phosphatase (ALP) activity. As a result, it could be developed as a prospective biomaterial for bone regeneration.
The click chemistry reaction is inspired by nature and boasts mild reaction conditions, and also has high specificity, rich yielding and a speedy reaction rate [46][47]. In particular, it is biorthogonal and widely used in cell therapy with few side reactions [48]. Cysteine is commonly grafted into peptides since its thiol group and alkenes groups are rarely found in nature. Such peptides are extensively used to crosslink the polymers possessed alkene groups (typically the norbornene groups) to form hydrogels via the thiol-ene photo-click chemistry reaction between the thiol group and the alkene groups with cytocompatible light initiation. The reaction, which is mediated by light, starts with radical initiation upon irradiation to form a thiyl radical [49]. Furthermore, the hydrogels are polymerized in a step-growth manner. As a result, the hydrogels exhibit a spatiotemporally controlled gelation behavior and excellent cell encapsulation ability [50]. MMP-sensitive PEG-based hydrogels were identified, and they were found to be formed via the click reaction between 4-arm PEG-modified with norbornene groups and MMP-cleavable crosslinker (KCGPQG↓IWGQCK) [34]. Cells and growth factors were co-encapsulated into the hydrogel and functioned well based on the biocompatibility of this polymer ( Figure 2 ).
This entry is adapted from the peer-reviewed paper 10.3390/gels7040199
References
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247.
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213.
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9.
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417.
- Shekaran, A.; Garcia, J.R.; Clark, A.Y.; Kavanaugh, T.E.; Lin, A.S.; Guldberg, R.E.; Garcia, A.J. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials 2014, 35, 5453–5461.
- Wang, J.; Youngblood, R.; Cassinotti, L.; Skoumal, M.; Corfas, G.; Shea, L. An injectable PEG hydrogel controlling neurotrophin-3 release by affinity peptides. J. Control Release 2021, 330, 575–586.
- Liu, H.; Wang, Y.; Cui, K.; Guo, Y.; Zhang, X.; Qin, J. Advances in Hydrogels in Organoids and Organs-on-a-Chip. Adv. Mater. 2019, 31, e1902042.
- Lee, H.P.; Gu, L.; Mooney, D.J.; Levenston, M.E.; Chaudhuri, O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017, 16, 1243–1251.
- Lathuiliere, A.; Cosson, S.; Lutolf, M.P.; Schneider, B.L.; Aebischer, P. A high-capacity cell macroencapsulation system supporting the long-term survival of genetically engineered allogeneic cells. Biomaterials 2014, 35, 779–791.
- Jiang, L.B.; Su, D.H.; Ding, S.L.; Zhang, Q.C.; Li, Z.F.; Chen, F.C.; Ding, W.; Zhang, S.T.; Dong, J. Salt-Assisted Toughening of Protein Hydrogel with Controlled Degradation for Bone Regeneration. Adv. Funct. Mater. 2019, 29, 1901314.
- Li, Y.; Rodrigues, J.; Tomas, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221.
- Kwon, M.Y.; Wang, C.; Galarraga, J.H.; Pure, E.; Han, L.; Burdick, J.A. Influence of hyaluronic acid modification on CD44 binding towards the design of hydrogel biomaterials. Biomaterials 2019, 222, 119451.
- Wang, M.; Chen, M.; Niu, W.; Winston, D.D.; Cheng, W.; Lei, B. Injectable biodegradation-visual self-healing citrate hydrogel with high tissue penetration for microenvironment-responsive degradation and local tumor therapy. Biomaterials 2020, 261, 120301.
- Lueckgen, A.; Garske, D.S.; Ellinghaus, A.; Mooney, D.J.; Duda, G.N.; Cipitria, A. Enzymatically-degradable alginate hydrogels promote cell spreading and in vivo tissue infiltration. Biomaterials 2019, 217, 119294.
- Raman, R.; Hua, T.; Gwynne, D.; Collins, J.; Tamang, S.; Zhou, J.L.; Esfandiary, T.; Soares, V.; Pajovic, S.; Hayward, A.; et al. Light-degradable hydrogels as dynamic triggers for gastrointestinal applications. Sci. Adv. 2020, 6, eaay0065.
- Wang, X.; Chen, S.; Wu, D.; Wu, Q.; Wei, Q.; He, B.; Lu, Q.; Wang, Q. Oxidoreductase-Initiated Radical Polymerizations to Design Hydrogels and Micro/Nanogels: Mechanism, Molding, and Applications. Adv. Mater. 2018, 30, e1705668.
- Xing, Y.; Cheng, E.; Yang, Y.; Chen, P.; Zhang, T.; Sun, Y.; Yang, Z.; Liu, D. Self-assembled DNA hydrogels with designable thermal and enzymatic responsiveness. Adv. Mater. 2011, 23, 1117–1121.
- Hu, J.; Zhang, G.; Liu, S. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem. Soc. Rev. 2012, 41, 5933–5949.
- Li, P.; Zhong, Y.; Wang, X.; Hao, J. Enzyme-Regulated Healable Polymeric Hydrogels. ACS Cent. Sci. 2020, 6, 1507–1522.
- English, M.A.; Soenksen, L.R.; Gayet, R.V.; de Puig, H.; Angenent-Mari, N.M.; Mao, A.S.; Nguyen, P.Q.; Collins, J.J. Programmable CRISPR-responsive smart materials. Science 2019, 365, 780–785.
- Badeau, B.A.; DeForest, C.A. Programming Stimuli-Responsive Behavior into Biomaterials. Annu. Rev. Biomed. Eng. 2019, 21, 241–265.
- Fan, C.; Shi, J.; Zhuang, Y.; Zhang, L.; Huang, L.; Yang, W.; Chen, B.; Chen, Y.; Xiao, Z.; Shen, H.; et al. Myocardial-Infarction-Responsive Smart Hydrogels Targeting Matrix Metalloproteinase for On-Demand Growth Factor Delivery. Adv. Mater. 2019, 31, e1902900.
- Lutolf, M.P.; Lauer-Fields, J.L.; Schmoekel, H.G.; Metters, A.T.; Weber, F.E.; Fields, G.B.; Hubbell, J.A. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. USA 2003, 100, 5413–5418.
- Wang, H.; Tibbitt, M.W.; Langer, S.J.; Leinwand, L.A.; Anseth, K.S. Hydrogels preserve native phenotypes of valvular fibroblasts through an elasticity-regulated PI3K/AKT pathway. Proc. Natl. Acad. Sci. USA 2013, 110, 19336–19341.
- Benton, J.A.; Fairbanks, B.D.; Anseth, K.S. Characterization of valvular interstitial cell function in three dimensional matrix metalloproteinase degradable PEG hydrogels. Biomaterials 2009, 30, 6593–6603.
- Usprech, J.; Romero, D.A.; Amon, C.H.; Simmons, C.A. Combinatorial screening of 3D biomaterial properties that promote myofibrogenesis for mesenchymal stromal cell-based heart valve tissue engineering. Acta Biomater. 2017, 58, 34–43.
- Dai, J.; Qiao, W.; Shi, J.; Liu, C.; Hu, X.; Dong, N. Modifying decellularized aortic valve scaffolds with stromal cell-derived factor-1alpha loaded proteolytically degradable hydrogel for recellularization and remodeling. Acta Biomater. 2019, 88, 280–292.
- Tibbitt, M.W.; Rodell, C.B.; Burdick, J.A.; Anseth, K.S. Progress in material design for biomedical applications. Proc. Natl. Acad. Sci. USA 2015, 112, 14444–14451.
- Garcia, A.J. PEG-maleimide hydrogels for protein and cell delivery in regenerative medicine. Ann. Biomed. Eng. 2014, 42, 312–322.
- Lin, C.C. Recent advances in crosslinking chemistry of biomimetic poly (ethylene glycol) hydrogels. RSC Adv. 2015, 5, 39844–398583.
- Ouyang, L.; Dan, Y.; Shao, Z.; Yang, S.; Yang, C.; Liu, G.; Duan, D. MMP-sensitive PEG hydrogel modified with RGD promotes bFGF, VEGF and EPC-mediated angiogenesis. Exp. Ther. Med. 2019, 18, 2933–2941.
- Metzger, S.; Blache, U.; Lienemann, P.S.; Karlsson, M.; Weber, F.E.; Weber, W.; Ehrbar, M. Cell-Mediated Proteolytic Release of Growth Factors from Poly(Ethylene Glycol) Matrices. Macromol. Biosci. 2016, 16, 1703–1713.
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014.
- Sridhar, B.V.; Brock, J.L.; Silver, J.S.; Leight, J.L.; Randolph, M.A.; Anseth, K.S. Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Adv. Healthc. Mater. 2015, 4, 702–713.
- De Long, S.A.; Moon, J.J.; West, J.L. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials 2005, 26, 3227–3234.
- Patterson, J.; Hubbell, J.A. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials 2010, 31, 7836–7845.
- Lin, C.C.; Boyer, P.D.; Aimetti, A.A.; Anseth, K.S. Regulating MCP-1 diffusion in affinity hydrogels for enhancing immuno-isolation. J. Control Release 2010, 142, 384–391.
- Nguyen, L.H.; Kudva, A.K.; Guckert, N.L.; Linse, K.D.; Roy, K. Unique biomaterial compositions direct bone marrow stem cells into specific chondrocytic phenotypes corresponding to the various zones of articular cartilage. Biomaterials 2011, 32, 1327–1338.
- Nguyen, L.H.; Kudva, A.K.; Saxena, N.S.; Roy, K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials 2011, 32, 6946–6952.
- Bertlein, S.; Brown, G.; Lim, K.S.; Jungst, T.; Boeck, T.; Blunk, T.; Tessmar, J.; Hooper, G.J.; Woodfield, T.B.F.; Groll, J. Thiol-Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Adv. Mater. 2017, 29, 1703404.
- Highley, C.B.; Song, K.H.; Daly, A.C.; Burdick, J.A. Jammed Microgel Inks for 3D Printing Applications. Adv. Sci. 2019, 6, 1801076.
- Van Hoorick, J.; Dobos, A.; Markovic, M.; Gheysens, T.; Van Damme, L.; Gruber, P.; Tytgat, L.; Van Erps, J.; Thienpont, H.; Dubruel, P.; et al. Thiol-Norbornene gelatin hydrogels: Influence of thiolated crosslinker on network properties and high definition 3D printing. Biofabrication 2020, 13, 015017.
- Van Hoorick, J.; Gruber, P.; Markovic, M.; Rollot, M.; Graulus, G.J.; Vagenende, M.; Tromayer, M.; Van Erps, J.; Thienpont, H.; Martins, J.C.; et al. Highly Reactive Thiol-Norbornene Photo-Click Hydrogels: Toward Improved Processability. Macromol. Rapid Commun. 2018, 39, e1800181.
- Guo, K.; Wang, H.; Li, S.; Zhang, H.; Li, S.; Zhu, H.; Yang, Z.; Zhang, L.; Chang, P.; Zheng, X. Collagen-Based Thiol-Norbornene Photoclick Bio-Ink with Excellent Bioactivity and Printability. ACS Appl. Mater. Interfaces 2021, 13, 7037–7050.
- Fraser, D.; Nguyen, T.; Benoit, D.S.W. Matrix Control of Periodontal Ligament Cell Activity Via Synthetic Hydrogel Scaffolds. Tissue Eng. Part A 2021, 27, 733–747.
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021.
- Yigit, S.; Sanyal, R.; Sanyal, A. Fabrication and functionalization of hydrogels through "click" chemistry. Chem. Asian J. 2011, 6, 2648–2659.
- McKay, C.S.; Finn, M.G. Click chemistry in complex mixtures: Bioorthogonal bioconjugation. Chem. Biol. 2014, 21, 1075–1101.
- Grim, J.C.; Marozas, I.A.; Anseth, K.S. Thiol-ene and photo-cleavage chemistry for controlled presentation of biomolecules in hydrogels. J. Control Release 2015, 219, 95–106.
- Anderson, S.B.; Lin, C.C.; Kuntzler, D.V.; Anseth, K.S. The performance of human mesenchymal stem cells encapsulated in cell-degradable polymer-peptide hydrogels. Biomaterials 2011, 32, 3564–3574.
