We previously reported that heparin-based nano-biomaterials produced by simple mixing of raw materials exhibit sustained protein release, and thereby used as a drug delivery carrier. In the present study, we modified the nano-biomaterials without employing any organic synthetic approach to retain the property as a cell-penetrating peptide facilitating protein delivery to cell. We examined whether the heparin-based nano-biomaterials have the ability to deliver exogenous proteins into cultured cells in vitro or into murine hepatocytes in vivo through intravenous injection to anesthetized mice. Consequently, we found that the transferred protein was accumulated in both cultured cells and in vivo hepatocytes.
- heparin/protamine particles
- hepatocyte
- intravenous injection
- lacZ protein
- nanoparticles
- protein delivery
- self-assembling
Heparin-based nano-biomaterials as a drug delivery carrier
We previously developed a novel heparin-based nano-biomaterial, referred to as “low-molecular-weight heparin/protamine particles (LHPPs)”, that are capable of interacting with nucleic acids (NAs), proteins, and cells to protect them from proteolytic enzymes and thermal damage [1]. LHPPs can be synthesized by simply mixing two types of polymer chains (heparin and protamine) bearing opposite charges as monodisperse fine particles (micro- and nanoparticles). Their synthesis does not depend on organic reactions, and is performed via electrostatic interactions. The LHPPs are proven to exhibit controlled release of proteins [2][3][4] and useful for retaining cells, which allows them to adapt to cell culture environments [5]. For example, we reported that local administration of LHPPs carrying recombinant fibroblast growth factor-2 (FGF-2), known as an angiogenic factor, resulted in the controlled release of FGF-2, finally causing angiogenesis at the injection site of a mouse [2][3].
An idea to develop improved-LHPPs (i-LHPPs) enabling protein delivery into cells
In contrast with the above-mentioned case, LHPPs themselves were impossible to introduce a protein of interest into a cell. If this process was successful, the utility of LHPPs should be greatly expanded because direct introduction of protein into a cell does not require protein biosynthesis such as transcription (mRNA synthesis from the foreign gene), translation of mRNA into protein, and post-translational modifications. However, in the case of protein delivery into a cell, maintaining the three-dimensional (3D) structure of the delivered protein itself is very important. If the 3D protein structure is destroyed and metamorphosed upon delivery into a cell, the protein will rapidly lose its function. In addition, the case of in vivo, naked proteins introduced are rapidly degraded by proteolytic enzymes ubiquitously present in a given organism. Notably, it has been reported that a protein fused to cell penetrating peptides (CPPs) exhibited an increased efficiency of transmembrane transport by directly passing through a lipid membrane [6]. Generally, CPPs have an amino acid composition that either contains a high relative abundance of positively charged amino acids. Therefore, we hypothesized that it might be possible to modify the surface of LHPPs through electrostatic interactions, because LHPPs have surface charge.
In this study, we examined whether a new protein carrier, which is termed “improved LHPPs (i-LHPPs)”, could be produced by aforementioned idea without organic synthesis, and whether the i-LHPPs could be used as protein carriers for delivering a protein into a cell in vitro and in vivo mouse hepatocyte via hydrodynamics-based gene delivery (HGD).
Successful protein delivery by i-LHPPs
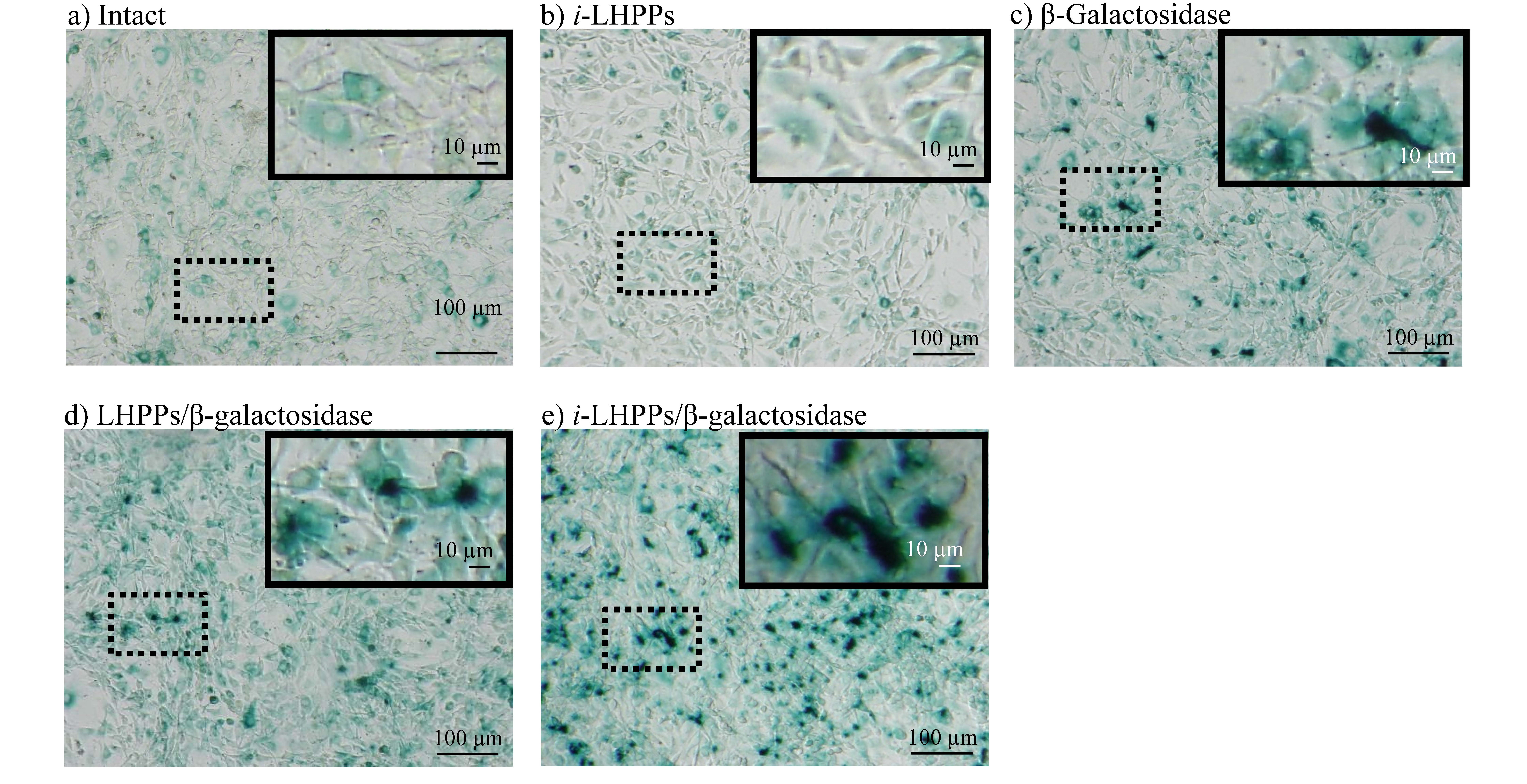
From examination of various conditions, we successfully developed i-LHPPs by mixing CPP with LHPPs [7]. To test whether i-LHPPs have the ability to transport a protein into a cell, NIH3T3 cells were co-incubated with medium containing i-LHPPs/β-galactosidase (β-gal) complex for 24 h. As expected, blue deposits derived from β-gal were more frequently discernible on the cells treated with i-LHPPs/β-gal complexes than those treated with β-gal alone or with LHPPs/β-gal complexes (Figure 1).
Figure 1. i-LHPPs-mediated protein delivery in vitro. (A) NIH3T3 cells stained in the presence of x-gal for β-galactosidase (β-gal) activity after in vitro protein delivery. In each microphotograph, the upper right image represents the magnified image of the dotted square area. Notably, in untreated (intact) cells (A-a), or cells treated with i-LHPPs alone (A-b), nonspecific positive reactions were observed, probably due to endogenous β-gal activity. A slight increase in blue deposition was noted in cells treated with β-gal alone (A-c), or in those treated with LHPPs/β-gal complexes (A-d). However, cells exposed to i-LHPPs/β-gal complexes exhibited distinct blue cytoplasmic deposits (A-e).
In addition, we used RNase T1 for i-LHPPs-mediated protein delivery experiment. RNase T1 is known to elicit cell death caused by inhibition of RNA synthesis when delivered inside a cell [8]. If the survival rate of cells in the intact group at each day of culture was defined as 100%, cells treated with the i-LHPPs/RNase T1 complex reached approximately 50% survival after 3 days in culture. These findings clearly suggest an incorporation of exogenous protein by a cell after i-LHPPs-mediated protein delivery.
Next, to examine whether i-LHPPs-based protein delivery is also possible in vivo, we introduced the i-LHPPs/β-gal complex intravenously using the HGD method, which is known to be effective for targeted delivery of NAs to hepatocytes [9]. Consequently, extensive and substantial brown deposits were recognizable throughout the liver specimens obtained after intravenous delivery of i-LHPPs/β-gal complexes. From these findings, it was found that in vivo delivery of proteins into hepatocytes is possible without loss of protein integrity when i-LHPPs are used as protein carriers.
Conclusion
We developed a novel protein carrier system, termed i-LHPPs, allowing delivery of a target protein into cells both in vitro and in vivo [7]. The most remarkable advantage of using i-LHPPs is that it does not require any organic synthetic process, which always require skilled techniques and advanced expertise. i-LHPPs are produced through “simple mixing of materials”. By replacing CPP with other positively charged short peptides such as receptor specific-peptides, it would be possible to deliver a protein into specific-tissues or organs. Thus, this system has great potential for use in various research fields, including animal biotechnology and biomedicine, although there are some subjects to be overcome in future, including physicochemical characterization.
References
- Masayuki Ishihara; Satoko Kishimoto; Shingo Nakamura; Yoko Sato; Hidemi Hattori; Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications.. Polymers 2019, 11, 672, 10.3390/polym11040672.
- Shingo Nakamura; Yasuhiro Kanatani; Satoko Kishimoto; Shin-Ichiro Nakamura; Chizuko Ohno; Takuya Horio; Fujita Masanori; Hidemi Hattori; Yoshihiro Tanaka; Tomoharu Kiyosawa; et al. Controlled release of FGF-2 using fragmin/protamine microparticles and effect on neovascularization. Journal of Biomedical Materials Research Part A 2009, 91, 814-823, 10.1002/jbm.a.32265.
- Shingo Nakamura; Masayuki Ishihara; Megumi Takikawa; Satoko Kishimoto; Susumu Isoda; Masanori Fujita; Masahiro Sato; Tadaaki Maehara; Attenuation of Limb Loss in an Experimentally Induced Hindlimb Ischemic Model by Fibroblast Growth Factor-2/Fragmin/Protamine Microparticles as a Delivery System. Tissue Engineering Part A 2012, 18, 2239-2247, 10.1089/ten.tea.2011.0741.
- Shingo Nakamura; Megumi Takikawa; Masayuki Ishihara; Takefumi Nakayama; Satoko Kishimoto; Susumu Isoda; Yuichi Ozeki; Masahiro Sato; Tadaaki Maehara; Delivery system for autologous growth factors fabricated with low-molecular-weight heparin and protamine to attenuate ischemic hind-limb loss in a mouse model. Journal of Artificial Organs 2012, 15, 375-385, 10.1007/s10047-012-0658-0.
- Shingo Nakamura; Satoko Kishimoto; Shin-Ichiro Nakamura; Masaki Nambu; Masanori Fujita; Yoshihiro Tanaka; Yasutaka Mori; Masahiro Tagawa; Tadaaki Maehara; Masayuki Ishihara; et al. Fragmin/protamine microparticles as cell carriers to enhance viability of adipose-derived stromal cells and their subsequent effect on in vivoneovascularization. Journal of Biomedical Materials Research Part A 2010, 92, 1614-1622, 10.1002/jbm.a.32506.
- Maciej Gagat; Wioletta Zielińska; Alina Grzanka; Cell-penetrating peptides and their utility in genome function modifications (Review).. International Journal of Molecular Medicine 2017, 40, 1615-1623, 10.3892/ijmm.2017.3172.
- Shingo Nakamura; Naoko Ando; Masayuki Ishihara; Masahiro Sato; Development of Novel Heparin/Protamine Nanoparticles Useful for Delivery of Exogenous Proteins In Vitro and In Vivo.. Nanomaterials 2020, 10, 1584, 10.3390/nano10081584.
- Shunji Yuki; Yoshitaka Kondo; Fuminori Kato; Masanari Kato; Norifusa Matsuo; Noncytotoxic ribonuclease, RNase T1, induces tumor cell death via hemagglutinating virus of Japan envelope vector. JBIC Journal of Biological Inorganic Chemistry 2004, 271, 3567-3572, 10.1111/j.0014-2956.2004.04293.x.
- F Liu; Y K Song; D Liu; Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Therapy 1999, 6, 1258-1266, 10.1038/sj.gt.3300947.