It is widely recognized that the assessment of animal welfare should include measures of positive emotional (affective) state. Existing behavioral and physiological indicators of a positive affective state frequently lack sensitivity, objectivity or are unsuitable in a production environment. Therefore, there is a need to develop new approaches to accurately and objectively measure a positive emotional state in animals, including novel molecular markers such a miRNA. These biomarkers must be measurable in the peripheral circulation and provide an accurate account of the physiological and molecular activity in regions of the brain associated with emotional processing. Further, such markers require validation against established behavioral and physiological indices. Here we investigated the efficacy of circulating miRNA as biomarkers of emotional state in the pig.
- welfare
- biomarkers
- positive affective state
- miRNA
- pigs
1. Introduction
2. Behaviour Data
2.1. Identification of Positive and Aversive Cue
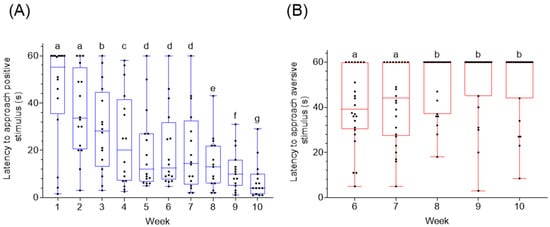
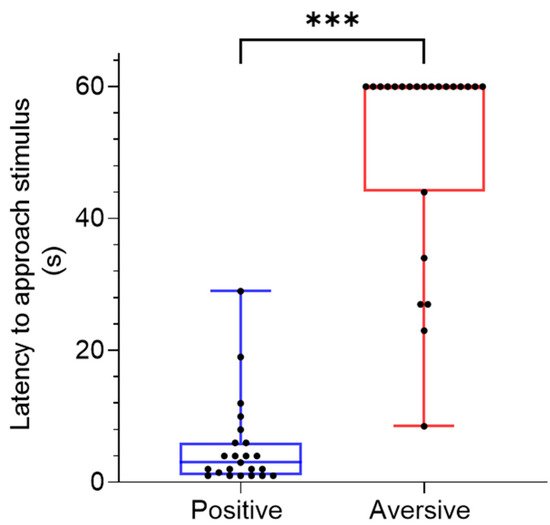
2.2. Cue Location and Latency to Approach
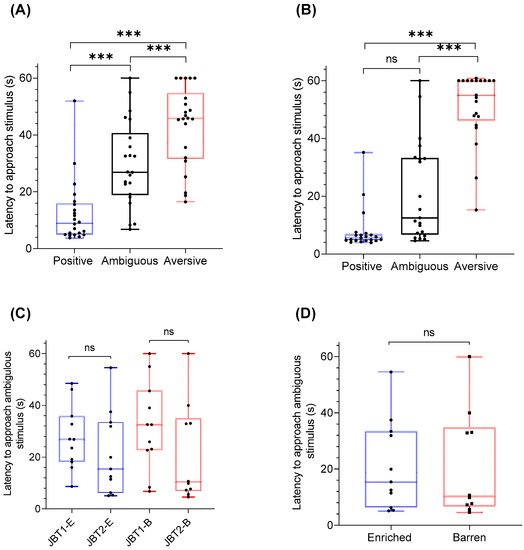
2.3. Treatment Effects on Judgment Bias
2.4 Blood and Brain MiRNA
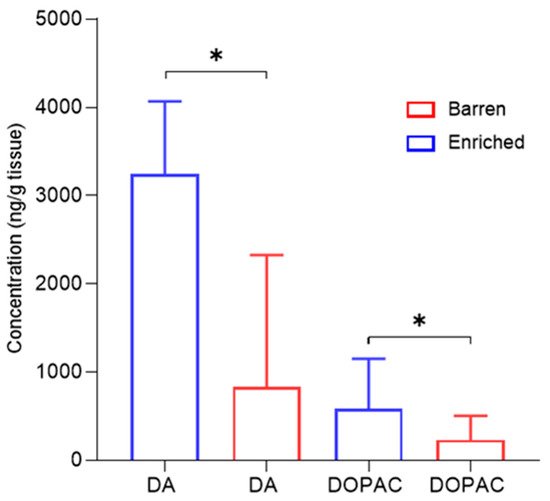
2.5. Dopamine, Serotonin and Metabolites
3. Discussion
This entry is adapted from the peer-reviewed paper 10.3390/ani11072054
References
- Mendl, M.; Burman, O.; Paul, E. An integrative and functional framework for the study of animal emotion and mood. Proc. R. Soc. B Biol. Sci. 2010, 277, 2895–2904.
- Schnall, S. Affect, mood and emotions. In Social and Emotional Aspect of Learning; Elsevier: Oxford, UK, 2010; pp. 59–64. ISBN 9780123814777.
- Paul, E.; Harding, E.; Mendl, M. Measuring emotional processes in animals: The utility of a cognitive approach. Neurosci. Biobehav. Rev. 2005, 29, 469–491.
- Mendl, M.; Burman, O.; Parker, R.; Paul, E. Cognitive bias as an indicator of animal emotion and welfare: Emerging evidence and underlying mechanisms. Appl. Anim. Behav. Sci. 2009, 118, 161–181.
- Mellor, D.; Beausoleil, N. Extending the ‘Five Domains’ model for animal welfare assessment to incorporate positive welfare states. Anim. Welf. 2015, 24, 241–253.
- Mason, G.; Mendl, M. Why is there no simple way of measuring animal welfare? Anim. Welf. 2013, 2, 301–319.
- Duncan, I. Science-based assessment of animal welfare: Farm animals. Rev. Sci. Tech. 2005, 24, 483–492.
- Kremer, L.; Klein Holkenborg, S.; Reimert, I.; Bolhuis, J.; Webb, L. The nuts and bolts of animal emotion. Neurosci. Biobehav. Rev. 2020, 113, 273–286.
- Roelofs, S.; Boleij, H.; Nordquist, R.; van der Staay, F. Making decisions under ambiguity: Judgment bias tasks for assessing emotional state in animals. Front. Behav. Neurosci. 2016, 10, 1–16.
- Bateson, M.; Emmerson, M.; Ergün, G.; Monaghan, P.; Nettle, D. Opposite effects of early-life competition and developmental telomere attrition on cognitive biases in juvenile European starlings. PLoS ONE 2015, 10, e132602.
- Whittaker, A.; Barker, T. A consideration of the role of biology and test design as confounding factors in judgment bias tests. Appl. Anim. Behav. Sci. 2020, 232, 105–126.
- George, R.P.; Barker, T.H.; Lymn, K.A.; Bigatton, D.A.; Howarth, G.S.; Whittaker, A.L. A Judgement Bias Test to Assess Affective State and Potential Therapeutics in a Rat Model of Chemotherapy-Induced Mucositis. Sci. Rep. 2018, 8, 8193.
- Doyle, R.; Fisher, A.; Hinch, G.; Boissy, A.; Lee, C. Release from restraint generates a positive judgment bias in sheep. Appl. Anim. Behav. Sci. 2010, 122, 28–34.
- Mendl, M.; Brooks, J.; Basse, C.; Burman, O.; Paul, E.; Blackwell, E.; Casey, R. Dogs showing separation-related behaviour exhibit a ‘pessimistic’ cognitive bias. Curr. Biol. 2010, 20, 839–840.
- Iyasere, O.; Beard, A.; Guy, J.; Bateson, M. Elevated levels of the stress hormone, corticosterone, cause ‘pessimistic’ judgment bias in broiler chickens. Sci. Rep. 2017, 7, 1–12.
- Douglas, C.; Bateson, M.; Walsh, C.; Bédué, A.; Edwards, S. Environmental enrichment induces optimistic cognitive biases in pigs. Appl. Anim. Behav. Sci. 2012, 139, 65–73.
- Scollo, A.; Gottardo, F.; Contiero, B.; Edwards, S. Does stocking density modify affective state in pigs as assessed by cognitive bias, behavioural and physiological parameters. Appl. Anim. Behav. Sci. 2014, 153, 26–35.
- Brajon, S.; Laforest, P.; Schmitt, O.; Devillers, N. The way humans behave modulates the emotional state of piglets. PLoS ONE 2015, 10, e133408.
- Lagisz, M.; Zidar, J.; Nakagawa, S.; Neville, V.; Sorato, E.; Paul, E.; Bateson, M.; Mendl, M.; Løvlie, H. Optimism, pessimism and judgment bias in animals: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 3–17.
- Bethell, E. A “how-to” guide for designing judgment bias studies to assess captive animal welfare. J. Appl. Anim. Welf. Sci. 2015, 18, 18–42.
- Si, Y.; Song, Z.; Sun, X.; Wang, J. microRNA and mRNA profiles in nucleus accumbens underlying depression versus resilience in response to chronic stress. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018, 177, 563–579.
- Wingo, A.; Almli, L.; Stevens, J.; Jovanovic, T.; Wingo, T.; Tharp, G.; Li, Y.; Lori, A.; Briscione, M.; Jin, P.; et al. Genome-wide association study of positive emotion identifies a genetic variant and a role for microRNAs. Mol. Psychiatry 2017, 22, 774–783.
- Whittaker, A.; Marsh, L. The role of behavioural assessment in determining positive affective states in animals. CAB Rev. 2019, 14, 1–13.
- Mellor, D. Animal emotions, behaviour and the promotion of positive welfare states. N. Z. Vet. J. 2012, 60, 1–8.
- Fonken, L.; Gaudet, A.; Gaier, K.; Nelson, R.; Popovich, P. MicroRNA-155 deletion reduces anxiety-and depressive-like behaviours in mice. Psychoneuroendocrinology 2016, 63, 362–369.
- Haramati, S.; Navon, I.; Issler, O.; Ezra-Nevo, G.; Gil, S.; Zwang, R.; Hornstein, E.; Chen, A. MicroRNA as repressors of stress-induced anxiety: The case of amygdalar miR-34. J. Neurosci. 2011, 31, 14191–14203.
- Lopez, J.; Lim, R.; Cruceanu, C.; Crapper, L.; Fasano, C.; Labonte, B.; Maussion, G.; Yang, J.; Yerko, V.; Vigneault, E.; et al. miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat. Med. 2014, 20, 764–768.
- Balakathiresan, N.; Chandran, R.; Bhomia, M.; Jia, M.; Li, H.; Maheshwari, R. Serum and amygdala microRNA signatures of posttraumatic stress: Fear correlation and biomarker potential. J. Psychiatr. Res. 2014, 57, 65–73.
- Rong, H.; Liu, T.; Yang, K.; Yang, H.; Wu, D.; Liao, C.; Hong, F.; Yang, H.; Wan, F.; Ye, X.; et al. MicroRNA-134 plasma levels before and after treatment for bipolar mania. J. Psychiatr. Res. 2011, 45, 92–95.
- Lai, C.; Yu, S.; Hsieh, M.; Chen, C.; Chen, H.; Wen, C.; Huang, Y.; Hsiao, P.; Hsiao, C.; Liu, C.; et al. MicroRNA expression aberration as potential peripheral blood biomarkers for schizophrenia. PLoS ONE 2011, 6, e21635.
- Wiegand, C.; Savelsbergh, A.; Heusser, P. MicroRNAs in psychological stress reactions and their use as stress-associated biomarkers, especially in human saliva. Biomed. Hub. 2017, 2, 1–15.
- Weber, J.; Baxter, D.; Zhang, S.; Huang, D.; Huang, K.; Lee, M.; Galas, D.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741.
- Tavares, G.; Torres, A.; de Souza, J. Early life stress and the onset of obesity: Proof of microRNAs’ involvement through modulation of serotonin and dopamine systems’ homeostasis. Front. Physiol. 2020, 11, 925.
- Dash, S.; Balasubramaniam, M.; Martínez-Rivera, F.; Godino, A.; Peck, E.; Patnaik, S.; Suar, M.; Calipari, E.; Nestler, E.; Villalta, F.; et al. Cocaine-regulated microRNA miR-124 controls poly (ADP-ribose) polymerase-1 expression in neuronal cells. Sci. Rep. 2020, 10, 11197.
- Baudry, A.; Mouillet-Richard, S.; Schneider, B.; Launay, J.; Kellermann, O. miR-16 targets the serotonin transporter: A new facet for adaptive responses to antidepressants. Science 2010, 329, 1537–1541.
- Kenny, P. Epigenetics, microRNA, and addiction. Dialogues Clin. Neurosci. 2014, 16, 335–344.
- Podolska, A.; Kaczkowski, B.; Kamp Busk, P.; Søkilde, R.; Litman, T.; Fredholm, M.; Cirera, S. MicroRNA expression profiling of the porcine developing brain. PLoS ONE 2011, 6, e14494.
- Lee, J.; Choi, H.; Heo, Y.; Chung, Y. Effect of floor space allowance on pig productivity across stages of growth: A field-scale analysis. Asian-Australas. J. Anim. Sci. 2016, 29, 739–746.
- Herskin, M.; Jensen, K. Effects of different degrees of social isolation on the behaviour of weaned piglets kept for experimental purposes. Anim. Welf. 2000, 9, 237–249.
- Puppe, B.; Ernst, K.; Schön, P.; Manteuffel, G. Cognitive enrichment affects behavioural reactivity in domestic pigs. Appl. Anim. Behav. Sci. 2007, 105, 75–86.
- Van de Weerd, H.; Day, J. A review of environmental enrichment for pigs housed in intensive housing systems. Appl. Anim. Behav. Sci. 2009, 116, 1–20.
- Tao, X.; Xu, Z.; Men, X. Analysis of serum microRNA expression profiles and comparison with small intestinal microRNA expression profiles in weaned piglets. PLoS ONE 2016, 11, e162776.
- Hao, Y.; Liu, J.; Zhang, Y.; Yang, P.; Feng, Y.; Cui, Y.; Yang, C.; Gu, X. The microrna expression profile in porcine skeletal muscle is changed by constant heat stress. Anim. Gen. 2016, 47, 365–369.
- Lecchi, C.; Zamarian, V.; Gini, C.; Avanzini, C.; Polloni, A.; Nodari, S.; Ceciliani, F. Salivary mirnas are potential biomarkers for the accurate and precise identification of inflammatory response after tail docking and castration in piglets. J. Anim. Sci. 2020, 98.
- Cools, R. Dopaminergic control of the striatum for high-level cognition. Curr. Opin. Neurobiol. 2011, 21, 402–407.
- Flagel, S.; Clark, J.; Robinson, T.; Mayo, L.; Czuj, A.; Willuhn, I.; Akers, C.; Clinton, S.; Phillips, P.; Akil, H. A selective role for dopamine in stimulus-reward learning. Nature 2011, 6, 53–57.
- Tottenham, N.; Galván, A. Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci. Biobehav. Rev. 2016, 70, 217–227.
- Gottfried, J. Neurobiology of Sensation and Reward; CRC Press: New York, NY, USA, 2011; pp. 348–349.
- Hensler, J. Serotonin in Mood and Emotion. Handb. Behav. Neurosci. 2010, 21, 367–378.
- Berger, M.; Gray, J.; Roth, B. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366.
- Brummelte, S.; Mc Glanaghy, E.; Bonnin, A.; Oberlander, T. Developmental changes in serotonin signaling: Implications for early brain function, behaviour and adaptation. Neuroscience 2017, 342, 212–231.
- Murray, E. The amygdala, reward and emotion. Trends Cogn. Sci. 2007, 11, 489–497.
- Dalley, J.; Everitt, B.; Robbins, T. Impulsivity, compulsivity, and top-down cognitive control. Neuron 2011, 24, 680–694.
- Puig, M.; Gulledge, A. Serotonin and prefrontal cortex function: Neurons, networks, and circuits. Mol. Neurobiol. 2011, 44, 449–464.
- Doyle, R.; Hinch, G.; Fisher, A.; Boissy, A.; Henshall, J.; Lee, C. Administration of serotonin inhibitor p-Chlorophenylalanine induces pessimistic-like judgment bias in sheep. Psychoneuroendocrinology 2011, 36, 279–288.
- Stracke, J.; Otten, W.; Tuchscherer, A.; Puppe, B.; Düpjan, S. Serotonin depletion induces pessimistic-like behaviour in a cognitive bias paradigm in pigs. Physiol. Behav. 2017, 174, 18–26.
- Rygula, R.; Papciak, J.; Popik, P. The effects of acute pharmacological stimulation of the 5-HT, NA and DA systems on the cognitive judgment bias of rats in the ambiguous-cue interpretation paradigm. Eur. Neuropsychopharmacol. 2014, 24, 1103–1111.
- Arroyo, L.; Carreras, R.; Valent, D.; Peña, R.; Mainau, E.; Velarde, A.; Sabrià, J.; Bassols, A. Effect of handling on neurotransmitter profile in pig brain according to fear related behaviour. Physiol. Behav. 2016, 1, 374–381.
- Ursinus, W.; Bolhuis, J.; Zonderland, J.; Rodenburg, T.; de Souza, A.; Koopmanschap, R.; Kemp, B.; Korte-Bouws, G.; Korte, S.; van Reenen, C. Relations between peripheral and brain serotonin measures and behavioural responses in a novelty test in pigs. Physiol. Behav. 2013, 118, 88–96.
- Whittaker, A.L.; Van Wettere, W.H.; Hughes, P.E. Space requirements to optimize welfare and performance in group housed pigs: A review. Am. J. Anim. Vet. Sci. 2012, 7, 48–54.
- Beattie, V.; O’Connell, N.; Kilpatrick, D.; Moss, B. Influence of environmental enrichment on welfare-related behavioural and physiological parameters in growing pigs. Anim. Sci. 2000, 70, 443–450.
- EU Council. Council Directive 2016/336/EC of 8 March 2016. Laying down Minimum Standards for the Protection of Pigs. Off. J. Eur. Union. 2016, pp. 5–13. Available online: https://eur-lex.europa.eu/eli/reco/2016/336/oj (accessed on 14 June 2021).
- Puppe, B.; Langbeina, J.; Bauer, J.; Hoyb, S. A comparative view on social hierarchy formation at different stages of pig production using sociometric measures. Livest. Sci. 2008, 113, 155–162.
- Docking, C.; Van de Weerd, H.; Day, J.; Edwards, S. The influence of age on the use of potential enrichment objects and synchronisation of behaviour of pigs. Appl. Anim. Behav. Sci. 2008, 110, 244–257.
- Bolhuis, J.; Schouten, W.; Schrama, J.; Wiegant, V. Behavioural development of pigs with different coping characteristics in barren and substrate-enriched housing conditions. Appl. Anim. Behav. Sci. 2005, 93, 213–228.
- Zupan, M.; Rehn, T.; de Oliveira, D.; Keeling, L. Promoting positive states: The effect of early human handling on play and exploratory behaviour in pigs. Animal 2016, 10, 135–141.
- Düpjan, S.; Ramp, C.; Kanitz, E.; Tuchscherer, A.; Puppe, B. A design for studies on cognitive bias in the domestic pig. J. Vet. Behav. 2013, 8, 485–489.
- Jensen, M.B. Effects of confinement on rebounds of locomotor behaviour of calves and heifers, and the spatial preferences of calves. Appl. Anim. Behav. Sci. 1999, 62, 43–56.
- Doyle, R.; Vidal, S.; Hinch, G.; Fisher, A.; Boissy, A.; Lee, C. The effect of repeated testing on judgment biases in sheep. Behav. Proc. 2010, 83, 349–352.
- Murphy, E.; Nordquist, R.; van der Staay, F. Responses of conventional pigs and Göttingen miniature pigs in an active choice judgment bias task. Appl. Anim. Behav. Sci. 2013, 148, 64–76.
- Barker, T.H.; Howarth, G.S.; Whittaker, A.L. Increased latencies to respond in a judgment bias test are not associated with pessimistic biases in rats. Behav. Proc. 2018, 146, 64–66.
