Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
Bamboos represent an emerging forest resource of economic significance and provide an avenue for sustainable development of forest resources. The development of the commercial bamboo industry is founded upon efficient molecular and technical approaches for the selection and rapid multiplication of elite germplasm for its subsequent propagation via commercial agro-forestry business enterprises.
- bamboo
- Dendrocalamus asper
- micropropagation
- plant tissue culture
- DNA barcoding
- genetic stability
1. Introduction
Bamboo is the fastest-growing flowering perennial grass and considered as one of the world’s most important tree species [1]. Bamboos belong to the largest family of grasses, the Poaceae (Gramineae), and constitute the Bambusoideae subfamily [2]. With 121 genera and 1662 species [3], the bamboo population can be divided into three zones geographically: the American zone, the Asian Pacific zone and the African zone [4], and according to reference [5], about 80% of bamboo forest lands and species in the world are distributed across the Asian Pacific region. Bamboo, in general, plays an important role in human life, mainly in terms of meeting the current economic, ecological, and human essential needs [6][7]. Several studies have shown that bamboos cultivated commercially are more renewable and sustainable than other woody plants, as the inefficient harvesting and use of bamboo has become a major focus worldwide [8][9]. A current report by reference [10] stated that the global demand for bamboo is expected to reach a revenue of USD 98.3 billion with a Computed Annual Growth Rate (CAGR) of 5% by 2025. The same study predicted that the biomass energy market will reach USD 98.0 billion by 2027 with a CAGR of 9.2% from 2020 to 2027. Since bamboo is undeniably the most important non-woody forest resource in Malaysia and some Southeast Asian countries, the traditional timber industry needs to develop the use of non-timber bamboo into a value-added material such as floorboards, building materials, composite boards and furniture, as well as biomass products [11].
2. Dendrocalamus asper
Taxonomically, Dendrocalamus belongs to the Bambuseae tribe and comprises about 35 species. In 2017, a study on Dendrocalamus and Bambusa conducted by reference [12] reported a higher similarity between these two genera when compared to other bamboo species. This finding supported the interpretation made by reference [13] that Dendrocalamus belongs to the same tribe as Bambusa. Besides, this similarity can be linked with their chromosome number, as with most species of the tropical bamboo genera, like Bambusa, Cephalostychum, Dendrocalamus, Gigantochloa and Melocanna reported to have a chromosome number of 72 (2n) [14]. D. asper, which is commonly referred to as sweet bamboo, is a multipurpose tropical clumping bamboo with high economic value [15][16]. Known also as rough bamboo, black bamboo or giant bamboo, D. asper grows to a height of 20–30 m, with a diameter of 8–20 cm and 20–45-cm-long internodes, and has relatively thick walls [17]. The origins of D. asper are not definitive, but according to reference [18], they are distributed across India and South East Asia, including Thailand, Vietnam, Malaysia, Indonesia and the Philippines. Recently, D. asper has been introduced in other tropical countries, including Ghana, Benin, DR Congo, Kenya and Madagascar. Figure 1 shows the distribution map of D. asper based on their endemic origin and subsequent introduction as an exotic species. Within tropical Asia, D. asper grows ideally in humid regions with rich, heavy soils, from lowlands to a 1500-m altitude, with an average annual rainfall of about 2400 mm. It can also survive well in semiarid environments with proper management. The mature stems are used to create furniture, musical instruments, household utensils, handicrafts and paper, while the upper internodes are used to make containers and cooking pots [19]. The tender young shoots are consumed as a vegetable and are thought to be the finest of all tropical Asiatic bamboos [20]. The rhizome, stems and branch cuttings can all be used to propagate D. asper. The propagules are grown in a nursery and then planted out in the field before or during the first half of the monsoon season after the roots have emerged. The best time to harvest stems is during the dry season; it is recommended to harvest mature stems that are 5–7 years old, while retaining some mature tillers in the clump.
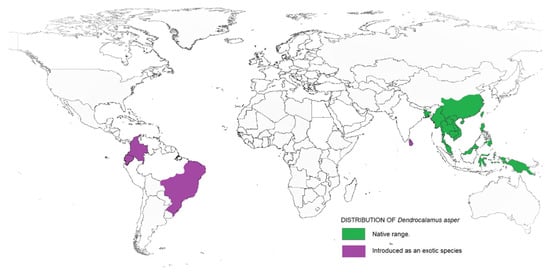
Figure 1. Range and distribution of D. asper in its native and introduced habitats.
3. Bamboo Propagation and Diseases
3.1. Traditional Propagation of Bamboos and Tissue Culture
Conventionally, bamboos are propagated through seeds. Short seed viability periods of 3–6 months, long-term gregarious flowering, the monocarpic nature of the plant, poor seed set and large-scale seed consumption by pests are all factors that restrict the use of seeds as a reliable resource of propagation [21][22]. Owing to the segregation of their traits, the genetic homogeneity of seed-based progenies is also in question. As a consequence, vegetative propagation from layering, off-set and rhizome planting, marcotting and branch and culm cuttings are used for propagating the bamboos [17][23][24]. The traditional bamboo propagation methods, on the other hand, are detrimental to mother plants during collection, involving high labor costs, transportation difficulties, bulky materials and seasonal dependence, which is typically limited to a short period of time, and these techniques are only effective for small-scale production [25][26]. The first report on a successful tissue culture of bamboo was done by reference [27], who described the embryo culture of D. strictus on a sucrose-enriched medium. In vitro propagation provides the ability to acquire large progenies from elite genotypes, since it was believed that it could solve most of the problems associated with conventional propagation [28]. In most cases, when designing protocols for in vitro plant propagation, trial-and-error experiments are needed to identify specific conditions for individual species, genotypes and even the donor plant development stages [29]. The aim of bamboo tissue culture regeneration protocols is to achieve the large-scale production of plants for operational planting, to produce disease-free and genetically uniform planting material and to provide material for breeding programs, as well as germplasm conservation [30].
3.2. Bamboo Diseases
Tissue culture has become a platform to confer resistance against specific diseases by manipulating the genetics of the plant systems. Bamboo tissue is composed primarily of Hemicellulose, Cellulose and Lignin [31], and microbes that rely on these as a source of carbon represent potential pathogens. Investigations carried out in China [32] in 148 bamboo species over a 11-year period from 1995 to 2006 recorded 208 potential pathogens, the majority of which were fungi (108). Similar studies carried out in Japan [33] reported 257 fungal strains, of which 75 could be identified using 18S rRNA gene and internal transcribed spacer region (ITS) analyses with Xylariales as the major dominant group. Bamboo died back, which was caused by the fungus Aciculosporium take, reported to predominantly infect Phyllostachys bambusoides, with a lower incidence in Phyllostachys pubescens Western Japan [34], leading to phenotypic changes referred to as the ”witches’ broom” of bamboo. Among the pathogens reported from India [35], Bambusa nutan was found to be infected by Nigrospora oryzae, the causative agent of leaf spot disease, whereas Fusarium oxysporum and F. verticillioides were dominant on Bambusa balcooa and D. asper, respectively. Another extensive study carried out in India across 12 phyla and 46 orders identified the pathogens belonging to the phylum Deuteromycota and Ascomycota as causative agents for foliage-related diseases. Basidiomycota was found to be associated with culm diseases, which is supported by the evidence that white rot fungi belong to this phylum and are involved actively in the degradation of lignin [36] and the utilization of carbohydrate complexes [37] that constitute the structural elements of bamboo. Interestingly, not all microbiota associated with bamboos have been reported to be pathogenic, with reports providing evidence of endophytic bacterial communities [38] associated with the rhizomes of tropical bamboos that share a unique symbiotic relationship and may also serve as a means of host defense [39] and biological control. Recent reports of the new fungal pathogen Arthrinium bambusicola in Thailand [40] and novel techniques such as high-throughput genome sequencing have provided new tools for the discovery and diagnosis of fungal pathogens [41], the early detection of which is critical to their control.
The Bamboo Mosaic Virus (BaMV) is one of the most well-documented and studied among the viruses associated with bamboos [42], although many individuals may be asymptomatic carriers with no physical evidence of viral infection. The mode of transmission of the virus appears to be mechanical injury, either via routine farming operations or insect vectors [43]. Recent reports have emerged of etic recombination events in Indonesia [44], which indicate Taiwan as the origin of the virus. Molecular data has provided evidence of the factors involved in the intracellular movement of the virus, which is mediated by movement proteins [45], and measurements for the containment and eradication of the BaMV have included treatments with abscisic acid [46], which has been reported to induce resistance and improve the host defense, as well as the application of interfering satellite RNA [47] in transgenic bamboo plants. The adoption of pertinent biosecurity measures during import of the germplasm, as well as the monitoring of invasive pathogens in commercial plantations, is currently the best available measure for the control of BaMV and other pathogens.
4. Regeneration of D. asper
In any plant tissue culture, choosing the appropriate propagation method is crucial. Different routes such as direct shoot induction (axillary shoot proliferation), the production of adventitious buds through organogenesis and somatic embryogenesis are pathways of choice for the rapid and large-scale propagation of bamboo using both juvenile and mature explants [48]. Reference [49] stated that callus have three basic developmental ways: somatic embryo development, shoot organ differentiation and a mixed development pathway that includes both somatic embryogenesis and shoot organogenesis. A developed in vitro culture of D. asper was successfully established from various explants. Seeds [50], mature plants of nodal explants [16][19][50][51][52][53][54][55], stem cuttings [56], small branch cuttings [57], nodal and leaf bases [58], inter node segments [59] and clump [60] were successfully used for the mass propagation of D. asper in vitro. Some researchers also used inflorescence explants for establishing protocols for the multiple shoot proliferation in D. asper [61] and in vitro flowering studies [62]. Most of the research available in D. asper used juvenile and mature tissues on enhanced axillary branching, with just a few reports on indirect organogenesis. By using nodal explants, the first regeneration of shoots and roots from D. asper callus was carried out by reference [51]. Moreover, several authors published studies incorporating both in vitro and in vivo multiplications of D. asper to improve the multiplication rate and measure the quality of plantlets in the field. Table 1 and Table 2 below respectively show the in vitro regeneration of D. asper through organogenesis and somatic embryogenesis using different explants. According to reference [29], because of the size and diversity of this plant family, establishing the best culture medium, combinations of plant growth regulators and other compounds in promoting the growth of explants will usually take several months. Therefore, to reduce the current gap between demand and supply, cost-effective methods for planting large-scale bamboo propagation in new bamboo forests must be established.
Table 1. Successful micropropagation of D. asper via organogenesis from various explants.
| Explant | Basal Medium | PGRs (as Indicated in μM Except Otherwise Mentioned) | Results | Reference(s) |
|---|---|---|---|---|
| Node (young first three segment) | MS | * BAP (22.0) ** BAP (22.0) + AdS (216.0) *** IBA (4.90) |
Shoot multiplication and rooting | [16] |
| Node | MS | * BAP (15.0) ** BAP (10.0) + AdS (75.0) *** ½ MS + IBA (5.0) + NAA (5.0) |
Shoot multiplication and rooting | [19] |
| Seeds, Nodes | MS | * BAP (13.32) ** BAP(13.32) ***NAA (16.11); IBA (49.0) |
Shoot multiplication and rooting | [50] |
| Node | MS | ** BAP (31.08) ***NAA (16.11) + IAA (5.71) |
Organogenesis, multiple shoots and rooting | [51] |
| Nodes | MS | ** BAP (13.32) + Ads (270.0) *** IBA (4.90) |
Shoot multiplication and rooting | [52] |
| Node | MS | * BAP (8.86) ** BAP (8.86) + Ads (13.5) + 3% Suc ***IBA (14.76) + NAA (3.67) + 3% Suc # 2,4-D (14.61) ##, ** 2,4-D (14.61) *** IBA (14.76) + NAA (3.67) |
Shoot multiplication and rooting | [53][59] |
| Node | MS | * ¼ MS BAP ** ¾ MS + 3 ppm Kn |
Shoot multiplication | [55] |
| Stem cuttings | MS | * BAP (0–8.88) + CW (0–20.0) ** BAP (22.2) *** NAA (2.68) + AA (283.5) + CA (130) + Cyst (206.25) |
Shoot multiplication and rooting | [56] |
| Small branch cuttings | MS | ** BA (3 × 10−5) | Shoot multiplication | [57] |
| Inter node segments | MS | * BAP (2.22) ** BAP (8.88) # Kin (23.25) + NAA (16.11) |
Shoot multiplication, rooting and callusing | [63] |
| Clump | MS | * BAP (15) ** mT (20) |
Shoot multiplication | [60] |
| Immature and mature inflorescence | MS | * BAP (31.08) ** BAP (13.32) *** IBA (49.0) |
Shoot multiplication and rooting | [61] |
| Seeds | MS | * BAP (22.2) ** BAP(1.332) * IBA (49.0) + NAA (16.11) |
Shoot multiplication and rooting | [64] |
| Seeds | MS | * BAP (22.2) ** BAP (13.32) *** IBA (49.0); NAA (16.11) |
Shoot multiplication and rooting | [65] |
| Seeds | MS | * BA (20.0) ** BA (10.0) *** IBA (40.0) |
Shoot multiplication and rooting | [66] |
| Node | MS | * TDZ (1.135) + NAA (1.34) + AA (283.5) + CA (130.0) + Cyst (206.25) ** TDZ (1.135) + NAA (1.34) + AA (283.5) + CA (130.0) + Cyst (206.25) *** ¼ MS + IBA (9.80) |
Shoot multiplication and rooting | [67] |
| In vitro grown shoots | MS | ** BAP (31.08) • NAA (16.11) + IBA (14.70) + 5% Suc |
Shoot multiplication and rhizogenesis | [68] |
| Seeds | MS | * BAP (13.32) ** BAP (13.32) *** IBA (34.30) |
Shoot multiplication and rooting | [69] |
AA, ascorbic acid; AdS, adenine sulphate; BAP, 6-Benzylaminopurine; CA, citric acid; CW, coconut water (milk); Cyst, cysteine; IAA, indole-3-acetic acid; IBA, indole-3-butyric acid; Kin, kinetin; MS, Murashige & Skoog medium; mT, Meta-topolin 6-(3-hydroxybenzylamino) purine; NAA, α-naphthaleneacetic acid; PGR, plant growth regulator; Suc, sucrose; TDZ, thidiazuron. *, Seed germination/shoot induction; **, Shoot proliferation/multiplication; ***, rooting; •, Rhizogenesis; #, callus initiation; ##, callus proliferation.
Table 2. The successful micropropagation of D. asper through somatic embryogenesis.
| Explant | Basal Medium | PGRs (as Indicated in μM Except Otherwise Mentioned) | Results | Reference(s) |
|---|---|---|---|---|
| Node | MS | # MMS + 2,4-D (30.0) * BAP (20.0) *** NAA (5.0–25.0) |
Somatic embryogenesis and germination | [54] |
| Nodal and leaf bases | MS | # 2,4-D (30.0) ## 2,4-D (9) + IAA (2.85) + BAP (0.88) X BAP (4.4) + GA3 (2.8) ** BAP (13.2) *** NAA (16) |
Somatic embryogenesis | [70] |
| Seeds | MS | #,## 2,4-D (13.59) or 2,4-D (2.265) *,** BAP (8.88) + NAA (2.68) + Kin (4.65) *** ½ MS + IBA (13.32) |
Somatic embryogenesis | [71] |
2,4-D, 2,4-dichlorophenoxyacetic acid; BAP, 6-Benzylaminopurine; GA, Gibberellic acid; IAA, indole-3-acetic acid; IBA, indole-3-butyric acid; Kin, kinetin; MS, Murashige & Skoog medium; MMS, Modified Murashige & Skoog medium; NAA, α-naphthaleneacetic acid; PGR, plant growth regulator; #, initiation of embryogenic callus; ##, embryogenic callus proliferation; X, plantlet development from somatic embryos *, Somatic embryo shoot induction; **, somatic embryo shoot proliferation/multiplication; ***, rooting.
5. Bamboo Dormancy and Bud Breaking
5.1. Seed Dormancy
The word “dormancy” refers to the temporary stop of plant growth. It comprises true dormancy, known as (“rest” or “endodormancy”) triggered by internal factors and climatic dormancy (“quiescence” or “ecodormancy”) controlled by external factors [72]. As mentioned by reference [73], dormancy and the breaking of dormancy in buds of bamboos vary with their position on the plant, the season of the year and the species, while seed dormancy is known to occur in many tropical tree species. In seeds, several methods are known to be involved in the induction of dormancy to the germinating state. In this section, the role of plant hormones, various treatments available are discussed for bamboo seed dormancy. Important factors influencing seed germination include the seed quality and their viability. Major causes linked to the loss of seed viability are the endogenous levels of auxins and abscisic acid (ABA) during prolonged storage [74]. Besides, bamboo seeds are short-lived, germinate within 3–7 days and the germination potential is season-dependent [75]. To preserve the viability for a longer period of time, seeds are usually stored at 4 °C in desiccators with anhydrous calcium chloride. Furthermore, reference [76] revealed that prechilling the seeds (4 to 5 °C) for 4 weeks could be the most effective way to extend their life. This process is known as vernalization, and it involves exposing seeds to low temperatures in order to stimulate or to enhance seed development [72]. For instance, reference [65] stored D. asper seeds at 4 °C for 3 months before undergoing surface sterilization. However, degradation can occur during storage. Depending on the predominant causes of dormancy, some authors [77][78][79] have suggested various approaches to break the seed dormancy in order to improve the germination rate and speed up the germination process. Besides, the breaking of seed dormancy varies from species to species. Therefore, it is very important to determine which method and condition are the best for each plant species. Various techniques are available that enhance the vigor of seeds, and these technologies are termed as seed invigoration/seed enhancement techniques [80]. Seed invigoration is a postharvest treatment that enhances seed production by ameliorating the germinability, storability and yield performance of the seeds [74]. Hydropriming, seed hardening, on-farm priming, osmo-priming, osmo-hardening, humidification, priming with plant growth regulators, polyamines, ascorbate, salicylate, ethanol, osmolytes, coating technologies and, more recently, pre-sowing dry heat treatments are some of the treatments used to invigorate seeds [75]. These strategies provide high-value crops with value-added solutions that improve the yield and quality. Generating greater emergence rates, rapid seedling growth and better stand developmental rates are the results of seeds priming [81]. However, no treatments have been applied to D. asper seeds in order to break their dormancy and improve their viability. In terms of plant growth regulators, reference [82] indicated that the major gibberellins formed by the germinating embryo are GA1 and GA3. Furthermore, GA3 and GA7 are thought to activate aleurone cells, and GA1 and GA4 are thought to regulate embryo development. GA2 and GA22 are two other active gibberellins, while others like GA12, GA17 and GA26 show no sign of reaction. The importance of endogenous GAs as a seed germination enhancer has also been earlier emphasized by reference [83]. When the seeds of D. membranaceus Munro were soaked in GA3 solution (50 ppm) overnight, a high percent of seed germination was stimulated, with a corresponding increase in shoot length (2.70 mm) and number of sprouts (7) per explant during culture initiation [84]. Similarly, reference [85] discovered that 0.5-mg/L GA3 supplemented in media promotes the germination of D. giganteus Munro seeds under light better than BAP and Kn. In addition, GA3 at 50 ppm was found to be the best pre-sowing treatment on D. hamiltonii seeds, with a statistically significant improvement in seed viability [75]. Furthermore, seed primed with 1% KNO3 solution increased the germination of D. strictus (Roxb.) by 80.4% at the fastest rate, and no mortality was recorded when transferred to soil [86]. However, reference [87] observed that osmopriming with KCl (10%) resulted in a maximum germination percentage of 83.1% when compared to KNO3 and PEG-6000 on D. strictus seeds. Meanwhile, reference [88] soaked the D. sinicus seeds in 0.5% (v/v) potassium permanganate (KMnO4) for 12 h and resulted in a high germination rate.
5.2. Bud Position on the Bamboo Plants
The position of explants was found to affect the culture initiation and the quality of the shoots formed under in vitro conditions. During in vitro bamboo propagation, the top and bottom portions of the nodal segment in culm bamboo can hardly regenerate. The initiation of the culture is more efficient when nodal segments from a healthy mature mother plant with disinfected lateral branches are used [19]. According to reference [89], the juvenility of lateral shoots, the season of the cultures initiated and the position of axillary bud on the branch highly affect the bud break frequency in D. longispathus. Moreover, reference [90] reported that nodal segments from mature clumps of B. bambos with pre-existing axillary buds were primarily preferred as explants due to their sufficient availability all-year-round to initiate in vitro cultures, while reference [91] reported that explants from young lateral buds showed a bud break in B. tulda. Besides, explants from healthy mother stock were found to be good for the regeneration of new plants in D. hamiltonii [92], P. stocksii Munro [93], G. angustifolia and D. giganteus [94]. Explants taken from higher branches were found to respond better to a multiplication medium with an early bud break than explants from lower branches [53]. In Arundinaria callosa, the position of the nodal buds in the lateral branches affected the efficiency of the bud breaks, resulting in a higher bud break when nodal explants are taken from the basal and middle nodes compared to the distal part of the secondary branches [95]. Reference [96] illustrated that the 5th–7th positions of B. nutans explants from the mother stock culm were the best for the maximum regeneration in the vitro culture in bud breaking, while reference [97] found the best regeneration for D. strictus taken from the 1st and 2nd positions of the base of the secondary branches. Similar findings have also been reported in D. longispathus [89] and B. vulgaris [98], which mid-culm nodes of secondary branches are in the best position for axillary shoot initiation explants. Furthermore, reference [69] stated that the best explants for axillary shoot proliferation in D. asper were taken from the mid-culm nodes of tertiary branches.
5.3. Season Collection of Explants
The period of explant collection for culture initiation was found to play an important role in reducing the level of contamination, increasing the bud break and increasing the number of shoots per explant [99]. The environmental conditions during different periods of the year varied the maturity status of the explants, hence influencing the response of explants to the culture initiation [69]. D. asper responded best to the culture conditions during the pre-monsoon season (May to June) but with a higher contamination rate [16], while references [19][99] stated that young branches (nodal segments) of D. asper collected in the spring (February–April) gave a better response in terms of lower contamination, early bud break and a higher number of shoots. On the other hand, reference [69] stated that the best time in initiating aseptic cultures for D. asper was in January and February, when the maximum bud break was achieved. In the spring, an increased cell division has been observed in trees as young buds produce auxins, which stimulates cell division in the cambium [100]. Moreover, the months of July–December were discovered to be unsuitable for optimal D. asper bud induction. Reference [19] found that during the rainy season (July–September), almost 50% of the contamination with moderate bud breaks was due to strong fungal and bacterial contaminants remaining underneath the leaf sheaths, while a poor response during the winter (October–December) was primarily due to the plant’s dormant and slow development. According to reference [69], the highest rate of contamination was also observed during the time of maximum rainfall (June–August). In a study of B. balcooa by reference [101], the explants collected during the rainy season in India (June–September) resulted in a high presence of contaminated explants. Furthermore, the establishment of B. oldhamii in vitro was a success when reference [102] collected the explant material by the end of the rainy season (June and July) in the Central-West Region of Brazil. Therefore, it is important to understand that bud break responsiveness is normally associated with the rainy season of different locations. Similar seasonal effects on bud breaks were also observed in D. giganteus and B. vulgaris [103], B. nutans [96][104], B. balcoa [105], D. hamiltonii Arn. Ex Munro [99] and B. Bambos [106].
6. Bamboo Genomics
Genome and transcriptome sequencing of commercially important plant species has led to the discovery of novel genes, the elucidation of biosynthetic pathways and the identification of genomic loci linked to quantitative traits. Bamboo occupies an important phylogenetic node in the grass family and the first attempt to compare the genomes of Oryza sativa and Zea mays was made by estimating the genome size of the tetraploid Moso bamboo (Phyllostachys pubescens) which was determined to be 2034 Mb following which, approximately 1000 genome survey sequence for the analysis of synteny [107]. Molecular markers from O. sativa were successfully applied and were able to resolve bamboo species into two major groups which concurred with the morphological classification as rhizome type, runner and clumper [108]. The first high quality of the draft genome of P. heterocycla var. Pubescens provided evidence of genome duplication and led to the identification of 31,967 genes [109]. The Bamboo genome database (Bamboo GDB) which has been developed as a direct result of multiple genome sequencing projects now provides researchers with a library of functionally annotated genes and pathways as well as tools for analysis and graphical representation of data sets [110]. Since then, transcriptome analysis of P. edulis has led to the discovery of genes linked to floral transition and flower development in bamboo, both of which are pertinent to the breeding industry [111]. The cumulative data provides an important resource for the development of molecular markers for the characterization of genome variation in bamboo via genome resequencing [112]. The wealth of information related to microsatellites has facilitated the reconstruction of high-resolution phylogenetic maps of bamboos [113]. The discovery of transposable genetic elements within the bamboo genome, which are responsible for somaclonal variation, has also provided insights into the phylogeny of Asian bamboos [114]. Transcription factors are important for the regulation of genes and their role in growth and development makes them of importance to genetic engineering, the characterization of these transcription factors in P. edulis has provided the foundation for the discovery and application of novel transcription factors for downstream applications in genetic modification [114]. The recent publication of the draft genome sequence of the diploid, herbaceous bamboo Raddia distichophylla (Schrad. ex Nees) Chase, has provide a clearer understanding of the process of lignification and the genes associated with this biosynthetic pathway [115]. The increase in the availability of both genome sequencing data from multiple projects when integrated with transcriptomic data from different developmental stages [116][117] will provide researchers and commercial breeders with data that can be applied for the improvement of bamboo via the application of Marker Assisted Breeding (MAS) program and genetic engineering of important regulatory pathways.
This entry is adapted from the peer-reviewed paper 10.3390/plants10091897
References
- Tanaka, E.; Tanaka, C.; Mori, N.; Kuwahara, Y.; Tsuda, M. Phenylpropanoid amides of serotonin accumulate in witches’ broom diseased bamboo. Phytochemistry. 2003, 64, 965–969.
- Yeasmin, L.; Ali, M.N.; Gantait, S.; Chakraborty, S. Bamboo: An overview on its genetic diversity and characterization. 3 Biotech 2015, 5, 1–11.
- Canavan, S.; Richardson, D.M.; Visser, V.; Roux, J.J.; Vorontsova, M.S.; Wilson, J.R. The global distribution of bamboos: Assessing correlates of introduction and invasion. AoB Plants 2016, 9, 78.
- Clark, L.G.; Londoño, X.; Ruiz-Sanchez, E. Bamboo taxonomy and habitat. In Bamboo, Tropical Forestry; Liese, W., Kohl, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 10, pp. 1–30.
- Mera, F.A.T.; Xu, C. Plantation management and bamboo resource economics in China. Cienc. Tecnol. 2014, 7, 1–12.
- Hoogendoorn, J.C.; Benton, A. 13 Bamboo and rattan production and the implications of globalization. In Forests and Globalization: Challenges and Opportunities for Sustainable Development; Nikolakis, W., Innes, J., Eds.; Routledge: London, UK, 2014; pp. 166–184.
- Liese, W.; Kohl, M. Bamboo: The Plant and Its Uses (Tropical Forestry); Springer: Basel, Switzerland, 2015; p. 356.
- Bansal, A.K.; Zoolagud, S.S. Bamboo composites: Material of the future. J. Bamboo Ratt. 2002, 1, 119–130.
- Song, X.; Zhou, G.; Jiang, H.; Yu, S.; Fu, J.; Li, W.; Wang, W.; Ma, Z.; Peng, C. Carbon sequestration by Chinese bamboo forests and their ecological benefits: Assessment of potential, problems, and future challenges. Environ. Rev. 2011, 19, 418–428.
- Grand View Research. Bamboos Market Size Worth $98.3 Billion by 2025|CAGR: 5.0%. 2020. Available online: https://www.grandviewresearch.com/press-release/global-bamboos-market (accessed on 20 August 2020).
- MTIB, Malaysian Timber Industry Board. Sabah Bamboo Industry Has Huge Potential, Sabah News, Daily Express. 2020. Available online: http://www.dailyexpress.com.my/news/155947/sabah-bamboo-industry-has-huge-potential (accessed on 8 October 2020).
- Konzen, E.R.; Peron, R.; Ito, M.A.; Brondani, G.E.; Tsai, S.M. Molecular identification of bamboo genera and species based on RAPD-RFLP markers. Silva Fenn. 2017, 51, 1–16.
- Kelchner, S.A.; Group, B.P. Higher level phylogenetic relationships within the bamboos (Poaceae: Bambusoideae) based on five plastid markers. Mol. Phylogenet. Evol. 2013, 67, 404–413.
- Thakur, A.; Barthwal, S.; Ginwal, H.S. Genetic diversity in bamboos: Conservation and improvement for productivity. In Bamboos in India, 3rd ed.; Shailendra, K., Singh, Y.P., Dinesh, K., Manisha, T., Santan, B., Eds.; ENVIS Centre on Forestry: Dehradun, India, 2016; Volume 3, pp. 131–146.
- Narin, C.; Kanokporn, P. Effect of thickness on qualities of dried sweet bamboo shoots (Dendrocalamus asper Backer) products. J. Food Health Bioenv Sci. 2021, 12, 1–8.
- Banerjee, M.; Gantait, S.; Pramanik, B.R. A two step method for accelerated mass propagation of Dendrocalamus asper and their evaluation in field. Physiol. Mol. Biol. Plants 2011, 17, 387–393.
- Hossain, M.A.; Kumar, S.M.; Seca, G.; Maheran, A.A.; Aini, A.S.N. Mass Propagation of Dendrocalamus asper By Branch Cutting. J. Trop. For. Sci. 2018, 30, 82–88.
- Benton, A. Priority Species of Bamboo. In Bamboo (Tropical Forestry); Liese, M., Kohl, M., Eds.; Springer: Cham, Switzerland, 2015; Volume 10, pp. 31–41.
- Singh, S.R.; Dalal, S.; Singh, R.; Dhawan, A.K.; Kalia, R.K. Micropropagation of Dendrocalamus asper Backer ex k. Heyne): An exotic edible bamboo. J. Plant Biochem. Biotechnol. 2012, 21, 220–228.
- Sowmya, C.; Jagadish, M.R.; Syam, V. Cultivation prospects of Dendrocalamus asper backer for edible shoots in semiarid and humid tropics of peninsular India. Int. J. Plant Anim. Environ. Sci. 2015, 5, 95–101.
- Mudoi, K.D.; Saikia, S.P.; Goswami, A.; Gogoi, A. Micropropagation of important bamboos: A review. Afr. J. Biotechnol. 2013, 12, 2770–2785.
- Ayana, A.D.A.; Tadesse, Z.; Kebede, Y.; Ayana, D.A. Effect of Storage Media and Storage Time on Germination and Field Emergence of Oxytenanthera abyssinica Seeds. Int. J. Basic Appl. Sci. 2012, 1, 218–226.
- Singh, S.; Kumar, P.; Ansari, S.A. A simple method for large-scale propagation of Dendrocalamus asper. Sci. Hortic. 2004, 100, 251–255.
- Seethalakshmi, K.; Jijeesh, C.M.; Unni, K.K. Traditional methods for bamboo propagation in nursery. In Proceedings of the International Conference on Improvement of Bamboo Productivity and Marketing for Sustainable Livelihood, New Delhi, Cane and Bamboo Technology Centre, Guwahati, India, 15–17 April 2008; pp. 28–36.
- Pasqualini, A.P.D.A.; Schneider, G.X.; Fraga, H.P.D.F.; Biasi, L.A.; Quoirin, M. In vitro establishment of Bambusa oldhamii Munro from fieldgrown matrices and molecular identification of endophytic bacteria. Pesqui. Agropecu. Trop. 2019, 49, e53673.
- Embaye, K.; Weih, M.; Ledin, S.; Christersson, L. Biomass and nutrient distribution in a highland bamboo forest in southwest Ethiopia: Implications for management. For. Ecol. Manag. 2005, 204, 159–169.
- Alexander, M.P.; Rao, T.C. In vitro culture of bamboo embryo. Curr. Sci. 1968, 37, 415.
- Nadha, H.K.; Salwan, R.; Kasana, R.C.; Anand, M.; Sood, A. Identification and elimination of bacterial contamination during in vitro propagation of Guadua angustifolia Kunth. Pharmacogn. Mag. 2012, 8, 93–97.
- Sandhu, M.; Wani, S.H.; Jiménez, V.M. In vitro propagation of bamboo species through axillary shoot proliferation: A review. Plant Cell Tissue Organ Cult. 2018, 132, 27–53.
- Gielis, J.; Peeters, H.; Gillis, K.; Oprins, J.; Debergh, P.C. Tissue culture strategies for genetic improvement of bamboo. Acta Hortic. 2001, 552, 195–204.
- Li, X.; Sun, C.; Zhou, B.; He, Y. Determination of Hemicellulose, Cellulose and Lignin in Moso Bamboo by Near Infrared Spectroscopy. Sci. Rep. 2015, 5, 17210.
- Xu, M.Q.; Dai, Y.C.; Fan, S.H.; Jin, L.X.; Lu, Q.; Tian, G.Z.; Wang, L.F. Records of bamboo diseases and the taxonomy of their pathogens in China (II). For. Res. 2007, 20, 45–52.
- Morakotkarn, D.; Kawasaki, H.; Seki, T. Molecular diversity of bamboo-associated fungi isolated from Japan. FEMS Microbiol. Lett. 2007, 266, 10–19.
- Hashimoto, Y.; Hattori, T.; Iwakiri, K.; Tamura, K.; Kuroda, A.; Sawada, Y. Current status of bamboo die back caused by the destructive disease ‘witches’-broom of bamboo’ in western Japan. Jpn. J. Conserv. Ecol. 2008, 13, 151–160.
- Sharada, P.; Nagaveni, H.C.; Remadevi, O.K.; Jain, S.H. Toximetric studies on some major bamboo pathogens. Indian For. 2013, 139, 814–820.
- Zhang, X.; Yu, H.; Huang, H.; Liu, Y. Evaluation of biological pretreatment with white rot fungi for the enzymatic hydrolysis of bamboo culms. Int. Biodeter. Biodegr. 2007, 60, 159–164.
- Xu, G.; Shi, Z.; Zhao, Y.; Deng, J.; Dong, M.; Liu, C.; Murugadoss, V.; Mai, X.; Guo, Z. Structural characterization of lignin and its carbohydrate complexes isolated from bamboo (Dendrocalamus sinicus). Int. J. Biol. Macromol. 2019, 126, 376–384.
- Singh, L.; Ruprela, N.; Dafale, N.; Thul, S.T. Variation in Endophytic Bacterial Communities Associated with the Rhizomes of Tropical Bamboos. J. Sustain. For. 2021, 40, 1–13.
- Shen, X.Y.; Cheng, Y.L.; Cai, C.J.; Fan, L.; Gao, J.; Hou, C.L. Diversity and antimicrobial activity of culturable endophytic fungi isolated from moso bamboo seeds. PLoS ONE 2014, 9, e95838.
- Tang, X.; Goonasekara, I.D.; Jayawardena, R.; Jiang, H.B.; Li, J.; Hyde, K.; Kang, J. Arthrinium bambusicola (Fungi, Sordariomycetes), a new species from Schizostachyum brachycladum in northern Thailand. Biodivers. Data J. 2020, 23, e58755.
- Geng, X.S.; Shu, J.P.; Peng, H.; Zhang, W. Fungal communities in twigs of three bamboo species based on high-throughput sequencing technology. Chin. J. Ecol. 2018, 37, 3493–3498.
- Meng, M.; Lee, C.C. Function and structural organization of the replication protein of Bamboo mosaic virus. Front. Microbiol. 2017, 8, 522.
- Chang, K.C.; Chang, L.T.; Huang, Y.W.; Lai, Y.C.; Lee, C.W.; Liao, J.T.; Lin, N.S.; Hsu, Y.H.; Hu, C.C. Transmission of bamboo mosaic virus in bamboos mediated by Insects in the order Diptera. Front. Microbiol. 2017, 8, 870.
- Abe, S.; Neriya, Y.; Noguchi, K.; Hartono, S.; Sulandari, S.; Somowiyarjo, S.; Natsuaki, T. First report of the complete genomic sequences from Indonesian isolates of bamboo mosaic virus and detection of genomic recombination events. J. Gen. Plant Pathol. 2019, 85, 158–161.
- Cheng, C.P. Host factors involved in the intracellular movement of Bamboo mosaic virus. Front. Microbiol. 2017, 8, 759.
- Alazem, M.; He, M.H.; Moffett, P.; Lin, N.S. Abscisic acid induces resistance against Bamboo mosaic virus through argonaute 2 and 3. Plant Physiol. 2017, 174, 339–355.
- Lin, K.Y.; Hsu, Y.H.; Chen, H.C.; Lin, N.S. Transgenic resistance to Bamboo mosaic virus by expression of interfering satellite RNA. Mol. Plant Pathol. 2013, 14, 693–707.
- Zhang, N.; Fang, W.; Shi, Y.; Liu, Q.; Yang, H.; Gui, R.; Lin, X. Somatic embryogenesis and organogenesis in Dendrocalamus hamiltonii. Plant Cell, Tissue Organ Cult. 2010, 103, 325–332.
- Che, P.; Lall, S.; Nettleton, D.; Howell, S.H. Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture. Plant Physiol. 2006, 141, 620–637.
- Arya, I.D.; Satsangi, R.; Arya, S. Rapid micropropagation of edible bamboo Dendrocalamus asper. J. Sustain. For. 2001, 14, 103–114.
- Ali, A.H.; Nirmala, C.; Badal, T.; Sharma, M.L. In-vitro organogenesis and simultaneous formation of shoots and roots from callus in Dendrocalamus asper. In Proceedings of the 8th World Bamboo Congress, Bangkok, Thailand, 16–18 September 2009; pp. 31–40.
- Kumar, V.; Singh, S.; Banerjee, M. Albino Regenerants Proliferation of Dendrocalamus asper in vitro. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 5027–5033.
- Nadha, H.K. In Vitro Clonal Propagation of Some Important Woody Bamboos and Ascertaining Their Clonal Fidelity. Ph.D. Thesis, Department of Biotechnology and Environmental Sciences, Thapar University, Patiala, India, 3 March 2013.
- Ojha, A.; Verma, N.; Kumar, A. In vitro micropropagation of economically important edible bamboo (Dendrocalamus asper) through somatic embryos from root, leaves and nodal segments expiants. Res. Crop. 2009, 10, 430–436.
- Gusmiaty; Restu, M.; Larekeng, S.H.; Setiawan, E. The optimization of in vitro micropropagation of betung bamboo (Dendrocalamus asper backer) by medium concentrations and plant growth regulators. IOP Conf. Ser. Earth Environ. Sci. 2020, 575, 12024.
- Suwannamek, A. In Vitro Culture of Dendrocalamus asper Backer. Master’s Thesis, Department of Horticulture, Faculty of Agriculture, Kasetsart University, Bangkok, Thailand, 1992.
- Vongvijitra, R. Traditional vegetative propagation and tissue culture of some Thai bamboos. In Proceedings of the International Bamboo Workshop, Conchin, India, 14–18 November 1988; pp. 148–150.
- Arya, S.; Satsangi, R.; Arya, I.D. Large scale plant production of edible bamboo Dendrocalamus asper through somatic embryogenesis. Bamboo Sci. Cult. 2008, 21, 21–31.
- Shroti, K.; Upadhyay, R.; Niratkar, C.; Singh, M. Micropropagation of Dandrocalamus asper through Inter Nodal Segment. Bull. Environ. Pharmacol. Life Sci. 2012, 1, 58–60.
- Ornellas, T.S.; Werner, D.; Holderbaum, D.F.; Scherer, R.F.; Guerra, M.P. Effects of Vitrofural, BAP and meta-Topolin in the in vitro culture of Dendrocalamus asper. Acta Hortic. 2015, 1155, 285–292.
- Arya, S.; Satsangi, R.; Arya, I.D. Direct regeneration of shoots from immature inflorescences in Dendrocalamus asper (edible bamboo) leading to mass propagation. Bamboo Soc. 2008, 21, 14–20.
- Satsangi, R.; Kalia, S.; Arya, I.D.; Arya, S. Flowering in exotic bamboo Dendrocalamus asper in India. Indian For. 2001, 127, 1053–1057.
- Nadha, H.K.; Rahul, K.; Sharma, R.K.; Anand, M.; Sood, A. In vitro propagation of Dendrocalamus asper and testing the clonal fidelity using RAPD and ISSR markers. Int. J. Curr. Res. 2013, 5, 2060–2067.
- Arya, I.D.; Arya, S. In vitro culture establishment of exotic bamboo Dendrocalamus asper. Indian J. Exp. Biol. 1997, 35, 1252–1255.
- Arya, S.; Sharma, S.; Kaur, R.; Arya, I.D. Micropropagation of Dendrocalamus asper by shoot proliferation using seeds. Plant Cell Rep. 1999, 18, 879–882.
- Semsuntud, N.; Ponoy, B.; Pattanaviboo, R.; Sathitviboo, R.; Nitiwatta-nachai, W.; Ramyangsi, S. Micropropagation of Dendrocalamus asper Backer. In Bamboo; Puangchit, L., Thaiutsi, B., Thamincha, S., Eds.; INBAR: Chiangmai, Thailand, 2000; pp. 81–93.
- Rathore, T.S.; Kabade, U.; Jagadish, M.R.; Somashekar, P.V.; Viswanath, S. Micropropagation and evaluation of growth performance of the selected industrially important bamboo species in southern India. In Proceedings of the 8th World Bamboo Congress, Bangkok, Thailand, 16–19 September 2009; pp. 41–55.
- Nirmala, C.; Ali, A.; Badal, T. De novo organogenesis in the form of rhizome in Dendrocalamus asper and D. membranaceus. Curr. Sci. 2011, 100, 468–470.
- Tuan, T.T.; Tu, H.L.T.; Giap, D.D.; Du, T.X. The increase in in vitro shoot multiplication rate of Dendrocalamus asper (Schult. f.) Back. ex Heyne. TAP CHI SINH HOC 2012, 34, 257–264.
- Arya, I.D.; Arya, S. In vitro shoot proliferation and somatic embryogenesis: Means of rapid bamboo multiplication. In Proceedings of the 10th World Bamboo Congress, Propagation, Plantation and Management, Damnyang, Korea, 17–22 September 2015.
- Zang, Q.; Lui, Q.; Zhuge, F.; Wang, X.; Lin, X. In Vitro Regeneration Via Callus Induction in Dendrocalamus asper (Schult.) Backer. Propag. Ornam. Plants 2019, 19, 66–71.
- Considine, M.J.; Considine, J.A. On the language and physiology of dormancy and quiescence in plants. J. Exp. Bot. 2016, 67, 3189–3203.
- Suwal, M.M.; Lamichhane, J.; Gauchan, D.P. Regeneration Technique of Bamboo Species through Nodal Segments: A Review. Nepal J. Biotechnol. 2020, 8, 54–68.
- Sharma, M.L.; Bala, N. Endogenous Levels of Plant Growth Substances in Seeds of Five Bamboo Species in Relation To Seed Viability. Indian J. Plant Physiol. 2006, 11, 358–363.
- Geetika, S.R.; Sharma, M.L. Effect of Pre-Sowing Invigouration Treatments on Performance of Ageing Dendrocalamus Hamiltonii Seeds. Int. J. Sci. Res. 2015, 4, 2277–8179.
- Chaiyarat, R. The effects of different treatments on seed germination and growth monastery Bamboo, Thyrsostachys siamensis. J. Bamboo Ratt. 2018, 17, 61–71.
- Airi, S.; Bhatt, I.D.; Bhatt, A.; Rawal, R.S.; Dhar, U. Variations in seed germination of Hippophae salicifolia with different presoaking treatments. J. For. Res. 2009, 20, 27–30.
- Azad, M.S.; Zedan-Al-Musa, M.; Matin, M.A. Effects of pre-sowing treatments on seed germination of Melia azedarach. J. For. Res. 2010, 21, 193–196.
- Azad, S.; Paul, N.K.; Matin, A. Do pre-sowing treatments affect seed germination in Albizia richardiana and Lagerstroemia speciosa? Front. Agric. China 2010, 4, 181–184.
- Moghadam, A.K.; Mohammadi, K. Different priming treatments affected germination traits of safflower. Appl. Sci. Rep. 2013, 2, 22–25.
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-Mediated Seed Priming Improves Germination, Growth, Yield, and Quality of Watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 2020, 10, 5037.
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307.
- Karssen, C.M.; Zagorski, S.; Kepczynski, J.; Groot, S.P.C. Key role for endogenous gibberellins in the control of seed germination. Ann. Bot. 1989, 63, 71–80.
- Brar, J.; Anand, M.; Sood, A. In vitro seed germination of economically important edible bamboo Dendrocalamus membranaceus Munro. Indian J. Exp. Biol. 2013, 51, 88–96.
- Devi, W.S.; Bengyella, L.; Sharma, G.J. In vitro seed germination and micropropagation of edible bamboo Dendrocalamus giganteus Munro using seeds. Biotechnology 2012, 11, 74–80.
- Sarkar, P.K.; Kumar, P.R.; Singh, A.K.; Bhatt, B.P. Effect of priming treatments on seed germination and seedling growth in bamboo . Acta Ecol. Sin. 2020, 40, 128–133.
- Singh, G.; Sharma, M.L. Effect of pre-sowing invigouration treatments on performance of ageing Dendrocalamus strictus seeds. Int. J. Adv. Res. 2015, 3, 1521–1526.
- Li, J.; Gao, C.; Miao, Y.; Liu, Z.; Cui, K. Development of a highly efficient callus induction and plant regeneration system for Dendrocalamus sinicus using hypocotyls as explants. Plant Cell Tissue Organ Cult. 2021, 145, 117–125.
- Saxena, S.; Bhojwani, S.S. In vitro clonal multiplication of 4-year-old plants of the bamboo, Dendrocalamus longispathus kurz. Vitr. Cell Dev. Biol. Plant 1993, 29, 135–142.
- Raju, R.I.; Roy, S.K. Mass propagation of Bambusa bambos (L.) Voss through in vitro culture. Jahangirnagar Univ. J. Biol. Sci. 2017, 5, 15–26.
- Pratibha, S.; Sarma, K.P. In vitro propagation of Bambusa tulda: An important plant for better environment. J. Environ. Res. Dev. 2013, 7, 1216–1223.
- Sood, A.; Ahuja, P.S.; Sharma, M.; Sharma, O.P.; Godbole, S. In vitro protocols and field performance of elites of an important bamboo Dendrocalamus hamiltonii nees Et Arn. Ex Munro. Plant Cell Tissue Organ Cult. 2002, 71, 55–63.
- Rathore, T.S.; Rai, V.R. Micropropagation of Pseudoxytenanthera stocksii munro. Vitr. Cell. Dev. Biol. Plant. 2005, 41, 333–337.
- Jiménez, V.M.; Guevara, E. Micropropagation of bamboo species through axillary shoot proliferation. In Protocols for Micropropagation of Woody Trees and Fruits; Jain, S.M., Häggman, H., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 465–476.
- Devi, W.S.; Sharma, G.J. In Vitro Propagation of Arundinaria callosa Munro—An Edible Bamboo from Nodal Explants of Mature Plants. Open Plant Sci. J. 2014, 3, 35–39.
- Kalpataru, D.M.; Siddhartha, P.S.; Mina, B. Effect of nodal positions, seasonal variations, shoot clump and growth regulators on micropropagation of commercially important bamboo, Bambusa nutans Wall. ex. Munro. Afr. J. Biotechnol. 2014, 13, 1961–1972.
- Chowdhury, P.; Das, M.; Sikdar, S.R.; Pal, A. Influence of the physiological age and position of the nodal explants on micropropagation of field-grown Dendrocalamus strictus nees. Plant Cell Biotechnol. Mol. Biol. 2004, 5, 45–50.
- Hirimburegama, K.; Gamage, N. Propagation of Bambusa vulgaris (yellow bamboo) through nodal bud culture. J. Hortic. Sci. 1995, 70, 4469–4475.
- Singh, S.R.; Dalal, S.; Singh, R.; Dhawan, A.K.; Kalia, R.K. Seasonal influences on in vitro bud break in Dendrocalamus hamiltonii Arn. ex Munro nodal explants and effect of culture microenvironment on large scale shoot multiplication and plantlet regeneration. Indian J. Plant Physiol. 2012, 17, 9–21.
- Funada, R.; Kubo, T.; Tabuchi, M.; Sugiyama, T.; Fushitani, M. Seasonal variations in endogenous indole-3-acetic acid and abscisic acid in the cambial region of Pinus densiflora Sieb. et Zucc. stems in relation to earlywood-latewood transition and cessation of tracheid production. Holzforschung 2001, 55, 128–134.
- Das, M.; Pal, A. In vitro regeneration of Bambusa balcooa Roxb.: Factors affecting changes of morphogenetic competence in the axillary buds. Plant Cell Tissue Organ Cult. 2005, 81, 109–112.
- Silveira, A.A.D.C.; Lopes, F.J.F.; Sibov, S.T. Micropropagation of Bambusa oldhamii Munro in heterotrophic, mixotrophic and photomixotrophic systems. Plant Cell Tissue Organ Cult. 2020, 141, 1–12.
- Ramanayake, S.M.S.D.; Yakandawala, K.; Deepika, P.K.D.N.; Ikbal, M.C.M. Studies on micropropagation of Dendrocalamus gigateus and Bambusa vulgaris var. striata. Popul. Environ. 1995, 1, 75–85.
- Thorpe, T.A.; Harvy, I.S.; Kumar, P.P. Application of micropropagation to forestry. In Micropropagation: Technology and Application; Debergh, P.C., Zimmerman, R.H., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; pp. 311–336.
- Mudoi, K.D.; Borthakur, M. In vitro micropropagation of Bambusa balcooa Roxb through nodal explants from field-grown culms and scope for upscaling. Curr. Sci. 2009, 96, 962–966.
- Anand, M.; Brar, J.; Sood, A. In Vitro Propagation of an Edible Bamboo Bambusa Bambos and Assessment of Clonal Fidelity through Molecular Markers. J. Med. Bioeng. 2013, 2, 257–261.
- Gui, Y.; Wang, S.; Quan, L.; Zhou, C.; Long, S.; Zheng, H.; Jin, L.; Zhang, X.; Ma, N.; Fan, L. Genome size and sequence composition of moso bamboo: A comparative study. Science in China Series C. Life Sci. 2007, 50, 700–705.
- Chen, S.Y.; Lin, Y.T.; Lin, C.W.; Chen, W.Y.; Yang, C.H.; Ku, H.M. Transferability of rice SSR markers to bamboo. Euphytica 2010, 175, 23–33.
- Peng, Z.; Lu, Y.; Li, L.; Zhao, Q.; Feng, Q.I.; Gao, Z.; Lu, H.; Hu, T.; Yao, N.; Liu, K.; et al. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat. Gen. 2013, 45, 456–461.
- Zhao, H.; Peng, Z.; Fei, B.; Li, L.; Hu, T.; Gao, Z.; Jiang, Z. BambooGDB: A bamboo genome database with functional annotation and an analysis platform. Database J. Biol. Databases Curation 2014, 2014, bau006.
- Gao, J.; Zhang, Y.; Zhang, C.; Qi, F.; Li, X.; Mu, S.; Peng, Z. Characterization of the floral transcriptome of Moso bamboo (Phyllostachys edulis) at different flowering developmental stages by transcriptome sequencing and RNA-seq analysis. PLoS ONE 2014, 9, e98910.
- Zhou, M.B.; Wu, J.J.; Ramakrishnan, M.; Meng, X.W.; Vinod, K.K. Prospects for the study of genetic variation among Moso bamboo wild-type and variants through genome resequencing. Trees 2019, 33, 371–381.
- Zhao, H.; Yang, L.; Peng, Z.; Sun, H.; Yue, X.; Lou, Y.; Dong, L.; Wang, L.; Gao, Z. Developing genome-wide microsatellite markers of bamboo and their applications on molecular marker assisted taxonomy for accessions in the genus Phyllostachys. Sci. Rep. 2015, 5, 8018.
- Li, S.; Ramakrishnan, M.; Vinod, K.K.; Kalendar, R.; Yrjälä, K.; Zhou, M. Development and Deployment of High-Throughput Retrotransposon-Based Markers Reveal Genetic Diversity and Population Structure of Asian Bamboo. Forests 2020, 11, 31.
- Liu, H.L.; Wu, M.; Li, F.; Gao, Y.M.; Chen, F.; Xiang, Y. TCP transcription factors in moso bamboo (Phyllostachys edulis): Genome-wide identification and expression analysis. Front. Plant Sci. 2018, 9, 1263.
- Li, W.; Shi, C.; Li, K.; Zhang, Q.J.; Tong, Y.; Zhang, Y.; Wang, J.; Clark, L.; Gao, L.Z. Draft genome of the herbaceous bamboo Raddia distichophylla. G3 2021, 11, jkaa049.
- Ma, X.; Zhao, H.; Yan, H.; Sheng, M.; Cao, Y.; Yang, K.; Xu, H.; Xu, W.; Gao, Z.; Su, Z. Refinement of bamboo genome annotations through integrative analyses of transcriptomic and epigenomic data. Comput. Struct. Biotechnol. J. 2021, 19, 2708–2718.
This entry is offline, you can click here to edit this entry!
