1. Introduction
Living organisms are open thermodynamic systems, critically relying on their energy metabolism for the maintenance of structural integrity and function. Energy homeostasis includes catabolic processes that start with the degradation of polysaccharides, lipids and proteins and lead to the production of macroergic compounds such as adenosine triphosphate (ATP). This universal macroergic compound used by living organisms, is produced via multistep anaerobic or aerobic oxidation processes.
Aerobic ATP production is accompanied by the generation of reactive oxygen species (ROS) as side products of operation of the mitochondrial electron transport chain [
1,
2]. Thus, besides producing energy-rich substances, the energy homeostasis generates potentially damaging side products such as ROS in the respiratory chain and methylglyoxal in glycolysis. Methylglyoxal and other α-dicarbonyl compounds can react with arginine and lysine residues of proteins, yielding glycated derivatives called advanced glycation end products (AGEs), while ROS can interact with virtually all components of living organisms, causing their modification [
3,
4,
5]. ROS are also produced by the metabolic systems not connected with energy production. In particular, monoamine oxidases, cytochromes P450, peroxisomal oxidases and plasma membrane-bound Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) can produce ROS [
6]. Since ROS discovery in living organisms in the early 1950th, they have been considered as damaging species [
7]. Therefore, most ROS studies focused on the investigation of their negative effects and protection against them [
8]. However, besides the damaging effects, at physiological concentrations mitochondrial and cytosolic ROS play important signaling roles in multiple cellular processes, including inflammation, cellular growth and differentiation [
9,
10,
11,
12]. Whether ROS effects are beneficial or detrimental depends on the balance between ROS generation and elimination as well as on the targets attacked [
11]. Usually, the antioxidant system of a young and adult organism copes with oxidative modifications of biomolecules but gradually loses this ability during aging. Therefore, aging is accompanied by an intensification of oxidative stress, a decreased efficiency of ATP production and the concomitant activation of the immune system [
3,
4,
13]. That is because both ROS and AGEs activate key proinflammatory molecules nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and NLR Family Pyrin Domain Containing 3 (NLRP3) (
Figure 1), causing enhanced production of proinflammatory cytokines [
14,
15,
16]. Inflammation, triggered in this way, leads to the production of different reactive species, particularly ROS (
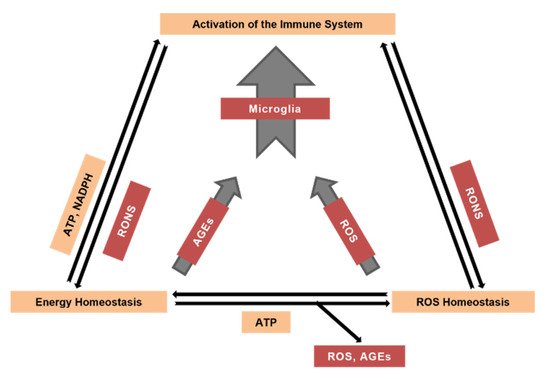
Figure 1), thus feeding back to energy and ROS homeostasis (Figure 2) [
17,
18]. While in a young organism byproducts generated by the energy metabolism are efficiently neutralized by the respective defense systems, these processes become imbalanced with advancing age [
4,
19].
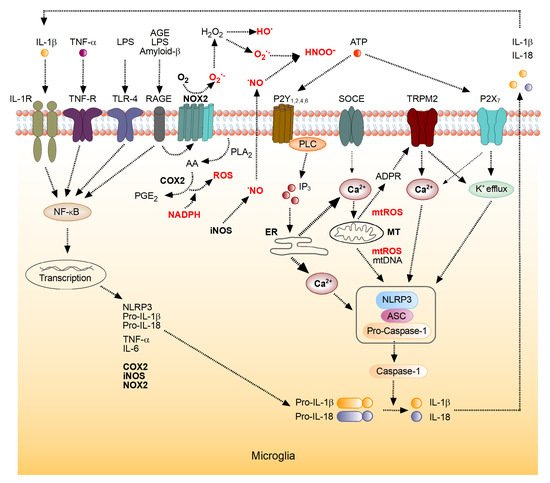
Figure 1. Molecular mechanisms underlying microglial reactive oxygen species (ROS) metabolism. Mechanisms involved in the interplay between the inflammation-mediated ROS production, increases in [Ca
2+]
i and cytokine production by microglia (see text for detailed description). IL-1β, interleukin 1β; TNF-α, tumor necrosis factor α; LPS, lipopolysaccharide; AGE, advanced glycation endproducts; IL-1R, IL-1β receptor; TNF-R, TNF-α receptor; TLR-4, Toll-Like Receptor 4; RAGE, a receptor for AGE; NADPH, a reduced form of nicotinamide adenine dinucleotide phosphate; NOX2, NADPH oxidase 2; P2Y
1,2,4,6, metabotropic ATP receptors, SOCE, store-operated Ca
2+ entry channel; TRPM2, Transient receptor potential cation channel, subfamily M, member 2; P2X
7, ionotropic ATP receptor, PLA
2, phospholipase A2; AA, arachidonic acid; COX2, cyclooxygenase 2; PGE
2, prostaglandin E2; iNOS, inducible NO-Synthase; PLC, Phospholipase C; IP
3, inositol 1,4,5-trisphosphate; ER, endoplasmic reticulum; MT, mitochondrion; mtROS, reactive oxygen species of mitochondrial origin; mtDNA, mitochondrial DNA; ADPR, ADP-ribose. The boxed structure consisting of NLRP3, adaptor protein ASC and Pro-Caspase-1 represents the NLRP3 inflammasome (modified from [
14,
16]).
The brain is especially prone to ROS-mediated toxicity for several reasons: (i) the intense oxidative metabolism, (ii) the high levels of polyunsaturated fatty acids, serving as primary substrates for ROS-promoted oxidation and (iii) a rather high number of resident immune cells [
20].
2. Mechanisms of ROS Generation
The bulk ROS amount in the organisms is generated by poorly controlled non-enzymatic processes such as autoxidation of small molecules (e.g., epinephrine, quinones), by better controlled enzymatic processes (e.g., operation of different oxidases) and by the escape of active electrons from electron transport chains [
11]. Under normoxic conditions, more than 90% ROS in eukaryotic cells are generated by mitochondria [
52], where above 95% of consumed oxygen is reduced via four-electron reduction of molecular oxygen by cytochrome oxidase [
53]. During the operation of mitochondria, however, some of the transported electrons escape ETC and join molecular oxygen giving rise to ROS. Besides the respiratory chain, ROS can be generated by multi-enzyme flavin-containing complexes such as alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase. Other enzymatic generators of ROS are monoamine oxidases located in the mitochondrial outer membrane. The latter way of ROS formation is especially important for neurons that produce and secrete biogenic amines [
6,
38]. Brain cyclooxygenases, lipoxygenases and NADPH oxidases also generate ROS. NADPH oxidases (NOXs) are often associated with immune cells like neutrophils and macrophages. However, they are also expressed in brain vascular endothelium and microglia and may contribute to age-related changes [
12,
14,
54]. These enzymes specifically produce either superoxide anion radical (O
2•−) or hydrogen peroxide (H
2O
2) or both, transferring electrons from NADPH to flavin adenine dinucleotide (FAD) first, and then—to heme of cytochrome
b558, and—from cytochrome
b558 to oxygen [
55]. Cyclooxygenases are heme-containing enzymes that convert arachidonic acid to prostaglandin H2. These enzymes can produce O
2•− since the catalysis occurs via the formation of transient carbon-centered radical species that, in turn, can react with molecular oxygen. Importantly, the formation of O
2•− by cyclooxygenases requires reducing co-substrates such as NADH or NADPH [
56,
57]. Superoxide is also produced in the brain endothelial cells by xanthine oxidase, a peroxisomal flavin-containing enzyme [
58].
In most cases where ROS are formed by enzymatic systems, sequential multistep one-electron O
2 reduction takes place. Interaction of one electron with molecular oxygen results in the formation of O
2•−. The latter can interact with one more electron and two protons forming H
2O
2. At the next reduction step, H
2O
2 can accept one more electron, resulting in the production of hydroxyl radical (HO
•) and hydroxyl anion (OH
−). Finally, HO
• may accept another electron and one proton, while OH
− may associate with a proton; in both cases, water molecules are formed. All three partially reduced oxygen intermediates, namely free radicals O
2•− and HO
• as well as H
2O
2 are called reactive oxygen species because they are more active than molecular triplet oxygen [
2,
59]. ROS-induced reactions occur spontaneously and are poorly controlled by living organisms.
All cells with aerobic metabolism possess a set of low and high molecular mass antioxidants [
11]. Low molecular mass antioxidants such as vitamins C and E, carotenoids, anthocyans and glutathione interact directly with any ROS type at low specificity. However, biological protection against HO
• is difficult, probably due to its high chemical activity, short lifespan and small diffusion distance, and thus organisms try to prevent HO
• production. In terms of high molecular mass antioxidants, levels of O
2•− and H
2O
2 are also controlled by enzymatic systems. Antioxidant enzymes form the so-called primary line, which directly deals with ROS, and a secondary line, which assists to the primary one and converts ROS-related components to less dangerous products. In addition, several enzymes regenerate low molecular mass antioxidants and repair certain types of oxidative damage [
6,
11]. Superoxide dismutases accelerate the conversion of O
2•− in H
2O
2 with concomitant formation of molecular oxygen, i.e., they dismutate one type of substrate molecules in two different products. Hydrogen peroxide can be eliminated by two types of enzymes: catalase, which dismutates H
2O
2 to water and molecular oxygen, and peroxidases, which use diverse co-substrates to reduce H
2O
2 to water and the respective oxidized co-substrate. Manganese-containing superoxide dismutase (Mn-SOD), specific glutaredoxins (Grx5), thioredoxins and peroxiredoxins (Prdx3 and Prdx5) scavenge ROS released to both, mitochondrial matrix and intermembrane space [
60]. In turn, O
2•− released by non-mitochondrial sources, such as NOX, cytochromes P450 and peroxisomal oxidases (for instance, xanthine oxidase) is scavenged by cytosolic copper-zinc-containing superoxide dismutase (Cu,Zn-SOD). Hydrogen peroxide, which is small and uncharged molecule, can easily cross lipid membranes. Whatever the place of H
2O
2 formation, it has a great chance to occur in the cytosol where it can be converted to water by peroxidases. Peroxidases use different cofactors and glutathione peroxidases are likely the best-studied enzymes of this group [
61]. They use the reductive power of glutathione that is oxidized to a dimeric form and may be further reduced by glutathione reductase at the expense of NADPH. The formed NADP
+ is then reduced by glucose-6-phosphate dehydrogenase, the key enzyme of the pentose phosphate pathway, that oxidizes glucose-6-phosphate to 6-phosphoglucolactone. This reaction finally connects ROS homeostasis with the catabolism of carbohydrates and overall energy-providing processes (
Figure 2). An imbalance between ROS generation and detoxification will eventually enhance the oxidation of important biomolecules and induce a loss of their functions. The systems responsible for the antioxidant defense, i.e., the elimination of oxidized molecules either by repair or biosynthesis wear out with cell’s age. This is especially relevant for neural tissue, which is predominantly composed of cells that rarely divide or regenerate.
Figure 2. The interplay between the energy/ROS homeostasis and the activation of the brain’s immune system during aging. RONS, reactive oxygen/nitrogen species.
3. The Role of the Brain’s Immune System in the Generation of ROS
Microglia, the main immune cells of the brain, vividly utilize ROS-mediated signaling under (patho)physiological conditions, and, consequently, possess several mechanisms for the generation of both intra- and extracellular ROS [
12,
14,
15]. As illustrated in
Figure 1, microglia express NADPH oxidases, capable of generating O
2•− and H
2O
2. Recent single-cell RNA sequencing analyses identified NADPH oxidase NOX2 as an isotype with the highest expression level in both human and mouse microglia [
12,
14]. In both species, the robust expression of NOX2 was seen not only during adulthood but also during development. NOX4 was also expressed in microglia, albeit at a much lower level than NOX2 [
12]. Besides NOX, NO synthases and cyclooxygenases are relevant sources of microglial ROS [
14]. For example, activation of microglia is associated with an NF-κB-dependent upregulation of iNOS and COX2 expression and a concomitant overproduction of intracellular ROS (
Figure 1). The intracellularly generated
•NO diffuses out of the cell and, besides acting as a secondary messenger, reacts with superoxide anion, generated by NOX2, forming peroxynitrite (ONOO
−). The latter is a highly reactive nitrogen species often causing tissue, cell and mitochondrial damage [
12,
62].
Like many other non-excitable cells, microglia utilize changes in the intracellular free Ca
2+ concentration ([Ca
2+]
i) for executing their sensor and effector functions [
63,
64,
65]. Such Ca
2+ signaling, mediated, for example, by activation of a plentitude of metabotropic receptors or store-operated Ca
2+ channels (
Figure 1) causes a release of ROS from mitochondria [
16,
66]. In turn, ROS produced in cytosol or mitochondria increase the production of ADP-ribose through degradation of poly-ADP-ribose in the nucleus or degradation of NAD
+ released from damaged mitochondria in the cytoplasm [
66,
67]. Together, ADP-ribose and Ca
2+ activate Ca
2+-permeant Transient Receptor Potential (TRPM2) channels (
Figure 1), known for their sensitivity to endogenous ROS [
67], thus further increasing [Ca
2+]
i. In this way, TRPM2 channels link ROS production to inflammasome activation in immune cells, where the expression of these channels is abundant.