Inspired by studies on outdoor organic aerosols (OA), recent studies discusses and prioritizes issues related to indoor water-soluble OA and their effects on human health, providing a basis for future research in the field. The following three main topics are addressed: (1) what is known about the origin, mass contribution, and health effects of water-soluble organic matter (WSOM) in outdoor air particles; (2) the current state-of-the-art on the WSOM in indoor air particles, the main challenges and opportunities for its chemical characterization and cytotoxicity evaluation; and (3) why the aerosol WSOM should be considered in future indoor air quality studies.
- organic aerosols
- water-soluble organic matter
- outdoor air particles
- indoor air chemistry
1. Introduction
Particulate matter (PM) of different sizes has been one of the most studied outdoor air pollutants. This interest in atmospheric PM is fueled by the realization of its negative impacts on air quality and human health, and large, but uncertain, effects on radiative climate forcing and atmospheric chemistry [1][2].
Over the past two decades, it has become also clear that an important fraction of airborne particulate organic matter (also known as organic aerosol, OA) is water-soluble. Observations made in Northern Hemisphere midlatitudes showed that water-soluble organic matter (WSOM) accounts for 10 to 80% of OA [3][4][5][6], whereas lower percentage values (up to 13%) have been reported for Southern Hemisphere locations [7]. The WSOM is ubiquitous in rural, urban, and marine aerosols, playing a key role in cloud formation and properties [8][9], Earth’s radiative balance [10][11], and atmospheric chemistry [10][12]. Dry and wet deposition of aerosol WSOM can also affect carbon and nitrogen biogeochemical cycles in aquatic ecosystems [13][14][15]. The WSOM in fine air particles may also exert adverse health effects by generating reactive oxygen and nitrogen species (e.g., [16][17]) or by promoting a moderate pro-inflammatory status [3].
In contrast to outdoor environments, the contribution, and chemical features of the WSOM of fine particles in indoor air remain poorly characterized. However, considering that secondary organic aerosols (SOA) contribute significantly to aerosol WSOM burden in outdoor PM 2.5 (e.g., [6]), and that indoor SOA can be a substantial fraction of indoor aerosols (e.g., [18]), one can hypothesize that the WSOM is an important component of indoor PM 2.5 . Moreover, it has been suggested that indoor SOA composition is dominated by highly oxygenated organic molecules [19], whose characteristics may resemble those of WSOM. Furthermore, recent measurements showed that total polar water-soluble organic gases (WSOGs) concentrations are much higher indoors than directly outdoors (on average 15 times higher, on a carbon-mass basis) [20]. The aqueous processing of these WSOGs can alter the composition of residential indoor air in ways that are not yet understood [20]. This current lack of knowledge in indoor aerosol WSOM warrants further attention as well as ascertaining whether this indoor OA fraction could plausibly induce adverse health effects. The outdoor studies can provide guidance on issues such as the characterization and cytotoxicity of WSOM in indoor environments.
2. Characteristics and Sources of Aerosol WSOM in Outdoor Environments
As mentioned earlier, the water-soluble OA fraction, generally measured as water-soluble organic carbon (WSOC), can contribute substantially to the aerosol carbon mass. Table 1 shows examples of ambient concentrations of organic carbon (OC), elemental carbon (EC), and WSOC in locations with different levels of pollution around the globe. The lowest WSOC concentrations are typically found in pristine locations, far from anthropogenic sources. Nevertheless, the WSOC is the most abundant carbon fraction at these locations; for example, for the Canadian High Arctic, it has been reported that the WSOC can account to 40–89% of the OC [21], and in the Colorado Rocky Mountains, more than 90% of the OC was found to be water soluble [22]. The origin of WSOC at these pristine locations include primary biological aerosol particles [21], long-range transported aerosols, and SOA formation via the photooxidation of anthropogenic and biogenic volatile organic compounds (VOCs) originating from either short- or long-term transport [21][22][23]. The Antarctic Peninsula is another a pristine location, where the analysis of WSOC in aerosol samples revealed fingerprints of three different sources: primary marine organics (i.e., sea spray), primary biological particles emitted from land biota and land vegetation (grasses, mosses, lichens), and SOA originating from VOCs emitted from algal communities colonizing the sea ice [24]. On average, outdoor studies worldwide have shown a decreasing gradient for the WSOC/OC ratios in the order rural/agricultural—suburban—urban—industrial, which reflects a less aged aerosol OC at the urban and industrial sites, although the contribution of secondary organics to the WSOC fraction cannot be disregarded (e.g., [5][7][25]). In fact, it has been reported that the WSOC/OC is higher in summer than in other seasons at a variety of locations, both urban and rural, with this feature associated with the strong photochemical processes due to higher sun radiation (e.g., [26][27]). Nevertheless, SOA associated with anthropogenic precursors (e.g., nitro-monoaromatic hydrocarbons (NMAHs), nitrated and oxygenated derivatives of polycyclic aromatic hydrocarbons (PAHs), and phthalic acids) can also contribute to the water-soluble OA load in urban locations, even in colder seasons [5][7][25][28]. This contribution can also change between urban locations; for example, Kitanovski et al. [28] reported that NMAH contribution to the so-called humic-like substances (HULIS) mass was higher in Thessaloniki (≈1.8%) than in Mainz (≈0.4%). Less oxidized OA originating from fossil fuel combustion emissions can also dominate in urban and industrial areas, which explains the low WSOC/OC ratios (0.3 to 0.4) and demonstrates that these anthropogenic emissions are not the main source of WSOC at these locations [7][27][29]. On the other hand, the temporal variations reported for the WSOC concentrations at both urban and industrial sites, with the highest values occurring at colder periods, have been associated with fresh and aged biomass combustion emissions. These results confirm the dominant contribution of these sources for the aerosol WSOC load in low temperature conditions [5][25][30].
| Sampling Site | OC (µg C m−3) |
EC (µg C m−3) |
WSOC (µg C m−3) |
Reference |
|---|---|---|---|---|
| Pristine | ||||
| Jungfraujoch, CH | 1.2 ± 0.4 | 0.19 ± 0.08 | 0.63 ± 0.23 | [31] |
| Alert, Canadian High Arctic | 0.07–0.39 | 0.04–0.30 | [21] | |
| Antarctic Peninsula | 0.07–0.14 | [24] | ||
| Colorado Rocky Mountains, US | 0.43 ± 0.24 | 0.04 ± 0.07 | 0.41 ± 0.27 | [22] |
| Chichijima Island, JP | 0.60–1.13 | 0.04 – 0.28 | 0.20–0.59 | [23] |
| Rural/Agricultural | ||||
| Rondônia, pasture site, BR | 2.2–39.6 | [32] | ||
| Moitinhos, agricultural, PT | 2.56–11.6 | 0.45–1.30 | 1.11–4.62 | [26] |
| Porto Velho, forest, MBB 1, BR | 0.03–0.10 | [7] | ||
| Porto Velho, forest, IBB 1, BR | 0.01–0.43 | |||
| Thompson Farm, US | 4.49 | 3.23 | [33] | |
| Sainte-Anne-de-Bellevue, Montreal, CA | 0.96 | [34] | ||
| Suburban | ||||
| Coruña, Summer, ES | 0.06–0.15 | [6] | ||
| Coruña, Winter, ES | 0.13–0.69 | |||
| Saitama, JP | 5.18–12.1 | 0.90–1.44 | 2.74–7.64 | [35] |
| Drummond, Montreal, CA | 1.19 | [34] | ||
| Urban | ||||
| Aveiro, PT | 4.99–8.22 | 0.13–1.10 | 1.81–3.12 | [5] |
| Lisbon, Summer, PT | 0.95–5.7 | [7] | ||
| Mainz, DE | 2.07 | [28] | ||
| Thessaloniki, GR | 4.20 | |||
| Helsinki, FI | 0.67–7.5 | 0.26–4.6 | [30] | |
| Rio de Janeiro, Summer, BR | 0.83–1.3 | [7] | ||
| São Paulo, BR | 0.07–0.45 | |||
| Medellín, Dry season, CO | 0.05–0.34 | |||
| Bogotá, Wet season, CO | 0.04–0.13 | |||
| Lima, Winter, PE | 0.27–0.38 | |||
| Buenos Aires, Winter, AR | 0.07–4.7 | |||
| Atlanta, Summer, US | 4.30 | 3.12 | [36] | |
| Little Rock, US | 0.7–5.9 | [25] | ||
| Anjou, Montreal, CA | 1.13 | [34] | ||
| Gwangju, Winter, KR | 2.5–17.7 | 0.7–8.5 | 1.2–6.7 | [37] |
| Beijing, CN | 3.4–36.7 | 1.6–10.6 | 1.3–14.7 | [29] |
| Industrial | ||||
| Lanzhou, Summer, CN | 3.1–59.5 | 1.5–24.5 | 1.2–13.2 | [27] |
| Saint-Jean-Baptiste, Montreal, CA | 1.26 | [34] | ||
As for the chemical features of aerosol WSOM in outdoor environments, this fraction covers a huge variety of molecular structures with different physicochemical properties, which reflects its different sources. To further enhance the diversity and structural complexity of this aerosol component, the chemical aging of primary OA during its transport can also be an important pathway for the formation of WSOM [38][39]. Therefore, it has become important to assess the structural and molecular characteristics of the WSOC to discriminate between primary OA and SOA because their quantitative contributions are a key aspect in evaluating air pollution control strategies. With the purpose of unravelling the complex structural composition of this aerosol component, several different offline and online analytical methodologies have been developed and applied by the outdoor research community [4][5][6][7][26][36][40][41][42][43][44][45][46][47]. Figure 1 summarizes the different state-of-the-art multidimensional analytical strategies currently available to quantify and characterize the atmospheric WSOM constituents and their various levels of identification.
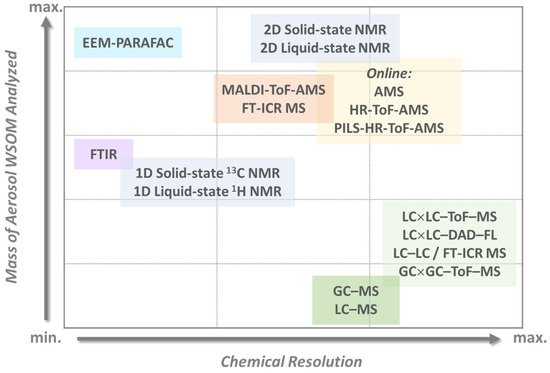
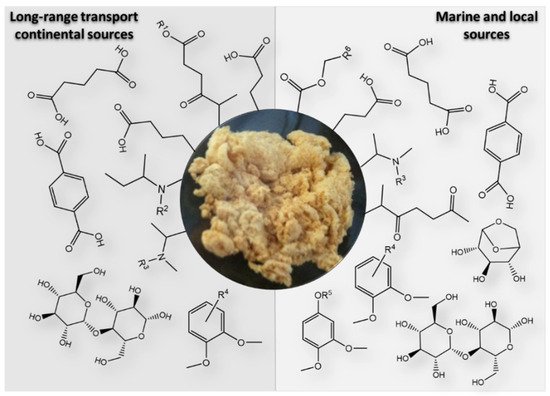
The studies published thus far have demonstrated that the aerosol WSOM fraction consists of a highly diverse suite of oxygenated compounds including dicarboxylic acids, keto-carboxylic acids, aliphatic aldehydes and alcohols, saccharides, saccharide anhydrides, aromatic acids, phenols, but also amines, amino acids, organic nitrates, and organic sulfates [7][26][40][45][48][49][50][51][52][53][54][55]. While this information on the major organic compound classes provides a good overview of the different types of potential functionalities within the complex organic mixtures, it is important to use these molecular-scale data to reconstruct the structural properties of the whole aerosol WSOC mass, for example, to infer on the physical properties of organic air particles. The recent work of Duarte et al. [41] is an example of how the compositional data of two urban aerosol WSOM samples collected in two short periods of time (one week each) under different wintry weather conditions was used to construct a structural model (presented in Figure 2 ) of the complex organic samples. The authors concluded that the studied urban aerosol WSOM exhibited three independent classes of compounds that varied both in content and molecular diversity: heteroatom-rich aliphatics (either chain or branched), carbohydrate-like moieties, and highly substituted aromatic units [41]. Despite the advances in understanding the atmospheric importance of the aerosol WSOM component, there are still knowledge gaps related to the complexity of its chemical composition, mechanisms of formation, atmospheric fate, and reactivity [56][11]. Furthermore, due to its dynamic nature, building a common model for the structure of the water-soluble fraction of OA is a challenging task [41], and further studies are needed by addressing additional aerosol WSOM sample sets from other locations (e.g., pristine, rural, agricultural) across different time scales [41]. Such a structural model would be of utmost importance because different chemical states are key factors determining the physical properties of OA.
3. Implications of WSOM to the Toxicity of Outdoor Air Particles
The atmospheric concentration of fine particulate matter (PM 2.5 ) has been recognized for decades as one of the most important risk factors associated with the adverse health effects of air pollution including cardiovascular impairments, respiratory diseases, and neurodevelopment deceleration, particularly in children [57][58][59]. Aside from the PM 2.5 mass, epidemiological studies have suggested the use of additional air quality metrics that are valuable in evaluating the health risks of inhalable air particles (e.g., [2][60][61]). In this regard, Riediker et al. [62] has published a very important review work highlighting the importance of the physicochemical properties of particles in producing toxicity, particularly those related to the particles’ surface reactivity, solubility, surface–tissue interactions, and biodurability. Parallel to this viewpoint on particle toxicology and health, it has been also demonstrated that the oxidative properties of some PM 2.5 constituents (e.g., transition metals, PAHs, bioaerosols (primary biological particles), levoglucosan (tracer of biomass burning), black carbon, and SOA) are at the base of the damage in cellular bio-macromolecules (e.g., plasmatic lipids, proteins, and DNA) [2][63][60][17][57][64][65][66][67][68][69]. The presence of free radicals in PM 2.5 (e.g., in combustion-derived particles), the transformation of aerosol organic constituents into reactive electrophilic metabolites (REMs) or the presence of transition metals, both inducing the production of intracellular reactive oxygen species (ROS) and the decrease of the cell’s antioxidant capacity (by the modification of the expression of antioxidant enzymes) due to PM 2.5 exposure, have been highlighted as important mechanisms of PM 2.5 -mediated toxicities [57][69]. As reviewed by Feng et al. [57] and Molina et al. [69], other potential mechanisms include: (a) metabolic activation, by the release of organics (e.g., PAHs) from PM 2.5 , which will be metabolically activated into REMs, thus causing toxic effects on cells; (b) mutagenicity/genotoxicity, caused by the presence of PAHs and/or nitro-compounds (i.e., nitro-PAHs and hydroxylamines) and transition metals in PM 2.5 ; and (c) inflammation, as a result of an increase in the gene expression and protein secretion of pro-inflammatory mediators due to PM 2.5 exposure.
As for the aerosol WSOM, which is usually taken as a measure of SOA formation, most of the studies have been focused on the contribution of this OA component to the ROS-generating potential of PM 2.5 [63][16][17][64][70][71][72][73][74][75]. The oxidative capacity of the aerosol WSOM has been commonly assessed by means of the cell-free assay dithiothreitol (DTT), which is highly sensitive to organic species present in atmospheric PM (e.g., [17][64][74][76]). It has been shown that ROS-activity is strongly influenced by the presence of WSOC in air particles, thus suggesting that SOA formation during transport/aging of OA in the atmosphere is a potential driver of ROS generation. Although this association between aerosol WSOC (and therefore, SOA) and ROS activity has been reported for both urban and non-urban locations, specific local sources and/or seasons might also contribute to the oxidative activity of water-soluble OA. For example, in an urban-traffic location, in the city of Thessaloniki, a significant correlation between WSOC and DTT activity was obtained for wintertime samples, suggesting the presence of a wintertime WSOC source, other than SOA formation, which contributes to the ROS activity in winter [17]. Biomass burning emissions (a known source of water-soluble OA, particularly in cold seasons) has been highlighted as a significant contributor to the oxidative potential of aerosol WSOC in the studied location [17][75]. In their recent review work, Rao et al. [64] also highlighted that the presence of a large amount of quinones in fresh wood smoke OA could be associated with an oxidative potential response. In the metropolitan Atlanta area, Fang et al. [74] also reported the important role of biomass burning to winter ROS activity of ambient water-soluble OA, whereas photochemical activity and SOA processing is likely to have a higher contribution to the summer ROS activity. Verma et al. [16] also concluded that SOA was the dominant source of ROS in summer as opposed to biomass burning in winter, leading to a significant seasonal variability in the oxidative activity of aerosol WSOM. Overall, the studies published thus far on this topic have showed that the ROS activity of the water-soluble organic fraction of outdoor air particles is likely to be driven by the relative dominance of aerosol WSOC sources at different locations and/or seasons (either primary water-soluble OA, chemical aging of OA, or SOA formation).
In the process of assessing which aerosol WSOM constituents contribute to the oxidative activity of this OA fraction, it has been shown that the most hydrophobic fraction of the WSOM—the so-called HULIS—is an active component generating ROS [70][71]. According to Lin and Yu [70], it is assumed that HULIS contains reversible redox sites (either quinone-like or nonquinone) that serve as electron carriers to catalyze the generation of ROS. If combined with redox-active water-soluble transition metals (e.g., Cu and Fe) [77], the most hydrophobic fraction of the water-soluble OA can further enhance the production of ROS [64][75][78], thus suggesting the need to further clarify the interaction mechanisms between the most hydrophobic WSOM and redox-active metals and their impacts on ROS activity.
Studies on the cytotoxicity and pro-inflammatory effects induced by aerosol WSOM are exceptionally limited [3][65][75]. Using human lung cell lines, Akhtar et al. [65] showed that urban quasi-ultrafine PM (aerodynamic diameter ≤ 0.2 µm), which was found to be enriched in WSOC, were capable of inducing a higher activation of antioxidant (heme oxygenase (HMOX-1) mRNA expression) and inflammatory (interleukin-8 ( IL-8) mRNA expression) responses. The authors further suggested that differences in composition within different PM size-fractions (coarse (2.5–10 µm) , fine (0.15–2.5 µm), and quasi-ultrafine) played an important role in the observed biological responses [65]. Velali et al. [75] also explored the in vitro cytotoxicity of the WSOC of size-segregated PM from two urban sites (traffic and urban background) in cold and warm periods. The authors reported that the cytotoxicity of PM peaked in the 0.49–0.97 µm size range, and correlated significantly with WSOC at both sites in the cold period [75]. Recently, Almeida et al. [3] investigated the relationship between the oxidative and pro-inflammatory potential of aerosol WSOM and their atmospheric concentrations and structural characteristics under different seasonal (autumn versus spring) and daily (day versus night) scenarios. The authors pinpointed a linkage between the compositional features of aerosol WSOM (namely at night, in autumn) and their ability to induce a moderate inflammatory status in macrophages. Furthermore, they also suggested that long-term exposure to very small amounts of WSOM may compromise the capacity of macrophages to respond to a subsequent inflammatory stimulus, which could result in increased susceptibility to respiratory infections [3]. It is obvious that the mechanisms by which outdoor aerosol WSOM may impair respiratory functions need to be further investigated as this would be beneficial for a better understanding of outdoor PM toxicity.
4. Water-Soluble Indoor Organic Aerosols: Challenges and Opportunities
The variability in the WSOM-to-WSOC conversion factor values recommended for outdoor OA studies should be noted, which highlights the importance of measuring the WSOM-to-WSOC ratios to reduce the uncertainties associated with the chemical mass closure assessment of atmospheric OA [5][26][31]. The use of such empirical conversion factor values is even more challenging in chemical mass balance studies of indoor air particles, and the reason is twofold: (1) not only do the WSOM-to-WSOC ratios depend on the class of organic compounds present in the WSOC component [79], and there is a clear lack of knowledge on the chemical composition of indoor particulate WSOC; (2) but also, the OC in indoor PM seems to be particularly unaffected by outdoor sources (e.g., [80][81]). Measurements and chemical characterization of the indoor WSOC fraction are, therefore, highly recommended to better ascertain the bulk chemical composition of indoor PM.
Almost two decades of WSOC measurements and characterization in outdoor air particles worldwide have highlighted the compositionally specific signatures associated with either chemical processing of OA or seasonal and emission source differences [43]. These compositional data have contributed greatly to our understanding of outdoor water-soluble OA and their effects on climate and human health. The indoor air chemistry community also has a long history with respect to the specific chemical species to be measured (mostly in the gas-phase) and their rationale, the analytical techniques to be employed in such measurements, and the most interesting indoor spaces to be studied (e.g., [82][83]). As recently reviewed by Abbat and Wang [83], indoor chemistry differs greatly from the outdoor chemistry, with the former being mostly driven by non-reactive partitioning and reactive processes that occur between gases, aerosol particles, and surfaces. The current understanding of indoor air chemistry and the nature of organic compounds in the indoor environment seems to be inevitably driven by the characterization according to volatility. Nonetheless, aerosol particles are also a dynamic player in indoor air chemistry [83][84], which means that the characterization of indoor aerosol composition, evolution, sources, and potential to affect human health is likewise highly needed.
As aforementioned, the WSOC fraction present in indoor PM is still poorly characterized, although there have been a few studies that have employed the WSOC as an indicator of SOA formation in indoor source apportionment studies [85][86][87][88]. Nonetheless, there is a certain level of uncertainty that one should be aware of when using the content of aerosol WSOC to perform indoor SOA estimates in a straightforward manner. First, the multiplication factor used to convert WSOC to SOA (a OM-to-OC ratio of 2.5) and the assumption that 20% of the SOA is water-insoluble OC [87]. Second, the aerosol WSOC can also have contributions from primary indoor emissions of water-soluble organics such as those from cleaning and other consumer products, cooking, and biomass burning [85][86][87]. Addressing these two issues alone should prompt a comprehensive investigation on the quantity and chemical features of the WSOC fraction present in indoor PM, considering different indoor spaces, occupancy purposes, and conditions as well as locations (e.g., urban, and rural). Furthermore, considering that indoor SOA formation can be a substantial fraction of indoor aerosols [18][89], and that indoor SOA composition is dominated by multifunctional and low-volatile highly oxygenated organic molecules [19][89], whose characteristics may resemble those of WSOC, more measurements of both WSOC and SOA composition indoors are warranted in order to improve the current knowledge on indoor air composition.
Another matter of debate is the oxidative aging of indoor OA, leading to changes in oxygen content and volatility, which may ultimately enhance the condensed-phase OA concentration [90]. Indoor chemical processes and chemical transformations have been highlighted as a research priority by the INDoor AIR POLLution NETwork (INDAIRPOLLNET), recently supported by the European Cooperation in Science and Technology (COST) [84]. Considering that for ambient air, the aging process in the atmosphere leads to the formation of increasingly oxidized, less volatile, more hygroscopic, and more water-soluble OA [91], it would be beneficial to consider the secondary formation and evolution of WSOC when predicting indoor OA concentrations. Recently, indoor WSOGs, comprising carbonyl compounds, carboxylic acids, epoxides, organic peroxides, organic nitrates, amines, and phenols, have been described as an ubiquitous and abundant component in residential indoor air, with potential implications on indoor air chemistry, air quality, and human health [20][92][93]. Nevertheless, little is still known about the concentration, composition, dynamics, fate, and health effects of WSOGs indoors. As it is quite possible that the indoor air composition of WSOGs is altered by aqueous chemistry reactions (in indoor surface films, skin or in wet particles) [20], one could argue that WSOGs will partition into the aqueous-phase and react further, thereby becoming less volatile and leading to the formation of condensed-phase secondary WSOC. This hypothesis is also critically needed to be assessed to predict its possible impact on indoor air composition and OA content.
This entry is adapted from the peer-reviewed paper 10.3390/app11219917
References
- Pöschl, U. Atmospheric Aerosols: Composition, Transformation, Climate and Health Effects. Angew. Chem. Int. Ed. 2005, 44, 7520–7540.
- Heal, M.R.; Kumar, P.; Harrison, R.M. Particles, air quality, policy and health. Chem. Soc. Rev. 2012, 41, 6606.
- Almeida, A.S.; Ferreira, R.M.P.; Silva, A.M.S.; Duarte, A.C.; Neves, B.M.; Duarte, R.M.B.O. Structural Features and Pro-Inflammatory Effects of Water-Soluble Organic Matter in Inhalable Fine Urban Air Particles. Environ. Sci. Technol. 2020, 54, 1082–1091.
- Lopes, S.P.; Matos, J.T.V.; Silva, A.M.S.; Duarte, A.C.; Duarte, R.M.B.O. 1 H NMR studies of water- and alkaline-soluble organic matter from fine urban atmospheric aerosols. Atmos. Environ. 2015, 119, 374–380.
- Duarte, R.M.B.O.; Freire, S.M.S.C.; Duarte, A.C. Investigating the water-soluble organic functionality of urban aerosols using two-dimensional correlation of solid-state 13C NMR and FTIR spectral data. Atmos. Environ. 2015, 116, 245–252.
- Duarte, R.M.B.O.; Piñeiro-Iglesias, M.; López-Mahía, P.; Muniategui-Lorenzo, S.; Moreda-Piñeiro, J.; Silva, A.M.S.; Duarte, A.C. Comparative study of atmospheric water-soluble organic aerosols composition in contrasting suburban environments in the Iberian Peninsula Coast. Sci. Total Environ. 2019, 648, 430–441.
- Duarte, R.M.B.O.; Matos, J.T.V.; Paula, A.S.; Lopes, S.P.; Pereira, G.; Vasconcellos, P.; Gioda, A.; Carreira, R.; Silva, A.M.S.; Duarte, A.C.; et al. Structural signatures of water-soluble organic aerosols in contrasting environments in South America and Western Europe. Environ. Pollut. 2017, 227, 513–525.
- Padró, L.T.; Tkacik, D.; Lathem, T.; Hennigan, C.J.; Sullivan, A.P.; Weber, R.J.; Huey, L.G.; Nenes, A. Investigation of cloud condensation nuclei properties and droplet growth kinetics of the water-soluble aerosol fraction in Mexico City. J. Geophys. Res. 2010, 115, D09204.
- Müller, A.; Miyazaki, Y.; Tachibana, E.; Kawamura, K.; Hiura, T. Evidence of a reduction in cloud condensation nuclei activity of water-soluble aerosols caused by biogenic emissions in a coolerate forest. Sci. Rep. 2017, 7, 1–9.
- Laskin, A.; Laskin, J.; Nizkorodov, S.A. Chemistry of Atmospheric Brown Carbon. Chem. Rev. 2015, 115, 4335–4382.
- Moise, T.; Flores, J.M.; Rudich, Y. Optical Properties of Secondary Organic Aerosols and Their Changes by Chemical Processes. Chem. Rev. 2015, 115, 4400–4439.
- George, C.; Ammann, M.; D’Anna, B.; Donaldson, D.J.; Nizkorodov, S.A. Heterogeneous Photochemistry in the Atmosphere. Chem. Rev. 2015, 115, 4218–4258.
- Iavorivska, L.; Boyer, E.W.; DeWalle, D.R. Atmospheric deposition of organic carbon via precipitation. Atmos. Environ. 2016, 146, 153–163.
- Witkowska, A.; Lewandowska, A.; Falkowska, L.M. Parallel measurements of organic and elemental carbon dry (PM1, PM2.5) and wet (rain, snow, mixed) deposition into the Baltic Sea. Mar. Pollut. Bull. 2016, 104, 303–312.
- Bao, H.; Niggemann, J.; Luo, L.; Dittmar, T.; Kao, S.J. Aerosols as a source of dissolved black carbon to the ocean. Nat. Commun. 2017, 8, 510.
- Verma, V.; Fang, T.; Xu, L.; Peltier, R.E.; Russell, A.G.; Ng, N.L.; Weber, R.J. Organic aerosols associated with the generation of reactive oxygen species (ROS) by water-soluble PM2.5. Environ. Sci. Technol. 2015, 49, 4646–4656.
- Samara, C. On the redox activity of urban aerosol particles: Implications for size distribution and relationships with organic aerosol components. Atmosphere 2017, 8, 205.
- Waring, M.S. Secondary organic aerosol in residences: Predicting its fraction of fine particle mass and determinants of formation strength. Indoor Air 2014, 24, 376–389.
- Kruza, M.; McFiggans, G.; Waring, M.S.; Wells, J.R.; Carslaw, N. Indoor secondary organic aerosols: Towards an improved representation of their formation and composition in models. Atmos. Environ. 2020, 240, 117784.
- Duncan, S.M.; Sexton, K.G.; Turpin, B.J. Oxygenated VOCs, aqueous chemistry, and potential impacts on residential indoor air composition. Indoor Air 2018, 28, 198–212.
- Fu, P.; Kawamura, K.; Chen, J.; Qin, M.; Ren, L.; Sun, Y.; Wang, Z.; Barrie, L.A.; Tachibana, E.; Ding, A.; et al. Fluorescent water-soluble organic aerosols in the High Arctic atmosphere. Sci. Rep. 2015, 5, 1–8.
- Xie, M.; Mladenov, N.; Williams, M.W.; Neff, J.C.; Wasswa, J.; Hannigan, M.P. Water soluble organic aerosols in the Colorado Rocky Mountains, USA: Composition, sources and optical properties. Sci. Rep. 2016, 6, 1–12.
- Boreddy, S.K.R.; Haque, M.M.; Kawamura, K. Long-term (2001–2012) trends of carbonaceous aerosols from a remote island in the western North Pacific: An outflow region of Asian pollutants. Atmos. Chem. Phys. 2018, 18, 1291–1306.
- Decesari, S.; Paglione, M.; Rinaldi, M.; Dall’osto, M.; Simó, R.; Zanca, N.; Volpi, F.; Cristina Facchini, M.; Hoffmann, T.; Götz, S.; et al. Shipborne measurements of Antarctic submicron organic aerosols: An NMR perspective linking multiple sources and bioregions. Atmos. Chem. Phys. 2020, 20, 4193–4207.
- Chalbot, M.-C.G.; Chitranshi, P.; Gamboa da Costa, G.; Pollock, E.; Kavouras, I.G. Characterization of water-soluble organic matter in urban aerosol by 1H-NMR spectroscopy. Atmos. Environ. 2016, 128, 235–245.
- Duarte, R.M.B.O.; Santos, E.B.H.; Pio, C.A.; Duarte, A.C. Comparison of structural features of water-soluble organic matter from atmospheric aerosols with those of aquatic humic substances. Atmos. Environ. 2007, 41, 8100–8113.
- Qin, J.; Zhang, L.; Zhou, X.; Duan, J.; Mu, S.; Xiao, K.; Hu, J.; Tan, J. Fluorescence fingerprinting properties for exploring water-soluble organic compounds in PM2.5 in an industrial city of northwest China. Atmos. Environ. 2018, 184, 203–211.
- Kitanovski, Z.; Shahpoury, P.; Samara, C.; Voliotis, A.; Lammel, G. Composition and mass size distribution of nitrated and oxygenated aromatic compounds in ambient particulate matter from southern and central Europe-implications for the origin. Atmos. Chem. Phys. 2020, 20, 2471–2487.
- Du, Z.; He, K.; Cheng, Y.; Duan, F.; Ma, Y.; Liu, J.; Zhang, X.; Zheng, M.; Weber, R. A yearlong study of water-soluble organic carbon in Beijing I: Sources and its primary vs. secondary nature. Atmos. Environ. 2014, 92, 514–521.
- Timonen, H.J.; Saarikoski, S.K.; Aurela, M.A.; Saarnio, K.M.; Hillamo, R.E.J. Water-soluble organic carbon in urban aerosol: Concentrations, size distributions and contribution to particulate matter. Boreal Environ. Res. 2008, 13, 335–346.
- Krivácsy, Z.; Gelencsér, A.; Kiss, G.; Mészáros, E.; Molnár, Á.; Hoffer, A.; Mészáros, T.; Sárvári, Z.; Temesi, D.; Varga, B.; et al. Study on the chemical character of water soluble organic compounds in fine atmospheric aerosol at the Jungfraujoch. J. Atmos. Chem. 2001, 39, 235–259.
- Graham, B.; Mayol-Bracero, O.L.; Guyon, P.; Roberts, G.C.; Decesari, S.; Facchini, M.C.; Artaxo, P.; Maenhaut, W.; Koll, P.; Andreae, M.O. Water-soluble organic compounds in biomass burning aerosols over Amazonia 1. Characterization by NMR and GC-MS. J. Geophys. Res. Atmos. 2002, 107.
- Ziemba, L.D.; Griffin, R.J.; Whitlow, S.; Talbot, R.W. Characterization of water-soluble organic aerosol in coastal New England: Implications of variations in size distribution. Atmos. Environ. 2011, 45, 7319–7329.
- Morera-Gómez, Y.; Cong, Z.; Widory, D. Carbonaceous Fractions Contents and Carbon Stable Isotope Compositions of Aerosols Collected in the Atmosphere of Montreal (Canada): Seasonality, Sources, and Implications. Front. Environ. Sci. 2021, 9, 1–18.
- Bao, L.; Sekiguchi, K.; Wang, Q.; Sakamoto, K. Comparison of water-soluble organic components in size-segregated particles between a roadside and a suburban site in Saitama, Japan. Aerosol Air Qual. Res. 2009, 9, 412–420.
- Sannigrahi, P.; Sullivan, A.P.; Weber, R.J.; Ingall, E.D. Characterization of water-soluble organic carbon in urban atmospheric aerosols using solid-state C-13 NMR spectroscopy. Environ. Sci. Technol. 2006, 40, 666–672.
- Cho, S.Y.; Park, S.S. Resolving sources of water-soluble organic carbon in fine particulate matter measured at an urban site during winter. Environ. Sci. Process. Impacts 2013, 15, 524–534.
- Maksymiuk, C.S.; Gayahtri, C.; Gil, R.R.; Donahue, N.M. Secondary organic aerosol formation from multiphase oxidation of limonene by ozone: Mechanistic constraints via two-dimensional heteronuclear NMR spectroscopy. Phys. Chem. Chem. Phys. 2009, 11, 7810–7818.
- Brege, M.; Paglione, M.; Gilardoni, S.; Decesari, S.; Cristina Facchini, M.; Mazzoleni, L.R. Molecular insights on aging and aqueous-phase processing from ambient biomass burning emissions-influenced Po Valley fog and aerosol. Atmos. Chem. Phys. 2018, 18, 13197–13214.
- Matos, J.T.V.; Duarte, R.M.B.O.; Lopes, S.P.; Silva, A.M.S.; Duarte, A.C. Persistence of urban organic aerosols composition: Decoding their structural complexity and seasonal variability. Environ. Pollut. 2017, 231, 281–290.
- Duarte, R.M.B.O.; Duan, P.; Mao, J.; Chu, W.; Duarte, A.C.; Schmidt-Rohr, K. Exploring water-soluble organic aerosols structures in urban atmosphere using advanced solid-state 13C NMR spectroscopy. Atmos. Environ. 2020, 230, 117503.
- Willoughby, A.S.; Wozniak, A.S.; Hatcher, P.G. Detailed source-specific molecular composition of ambient aerosol organic matter using ultrahigh resolution mass spectrometry and 1H NMR. Atmosphere 2016, 7, 79.
- Duarte, R.M.B.O.; Matos, J.T.V.; Duarte, A.C. Multidimensional analytical characterization of water-soluble organic aerosols: Challenges and new perspectives. Appl. Sci. 2021, 11, 2539.
- Decesari, S.; Mircea, M.; Cavalli, F.; Fuzzi, S.; Moretti, F.; Tagliavini, E.; Facchini, M.C. Source Attribution of Water-Soluble Organic Aerosol by Nuclear Magnetic Resonance Spectroscopy. Environ. Sci. Technol. 2007, 41, 2479–2484.
- Duarte, R.M.B.O.; Silva, A.M.S.; Duarte, A.C. Two-Dimensional NMR Studies of Water-Soluble Organic Matter in Atmospheric Aerosols. Environ. Sci. Technol. 2008, 42, 8224–8230.
- Matos, J.T.V.; Freire, S.M.S.C.; Duarte, R.M.B.O.; Duarte, A.C. Natural organic matter in urban aerosols: Comparison between water and alkaline soluble components using excitation–emission matrix fluorescence spectroscopy and multiway data analysis. Atmos. Environ. 2015, 102, 1–10.
- Matos, J.T.V.; Freire, S.M.S.C.; Duarte, R.M.B.O.; Duarte, A.C. Profiling Water-Soluble Organic Matter from Urban Aerosols Using Comprehensive Two-Dimensional Liquid Chromatography. Aerosol Sci. Technol. 2015, 49, 381–389.
- Paglione, M.; Saarikoski, S.; Carbone, S.; Hillamo, R.; Facchini, M.C.; Finessi, E.; Giulianelli, L.; Carbone, C.; Fuzzi, S.; Moretti, F.; et al. Primary and secondary biomass burning aerosols determined by proton nuclear magnetic resonance (1H-NMR) spectroscopy during the 2008 EUCAARI campaign in the Po Valley (Italy). Atmos. Chem. Phys. 2014, 14, 5089–5110.
- Wozniak, A.S.; Willoughby, A.S.; Gurganus, S.C.; Hatcher, P.G. Distinguishing molecular characteristics of aerosol water soluble organic matter from the 2011 trans-North Atlantic US GEOTRACES cruise. Atmos. Chem. Phys. 2014, 14, 8419–8434.
- Ng, N.L.; Canagaratna, M.R.; Zhang, Q.; Jimenez, J.L.; Tian, J.; Ulbrich, I.M.; Kroll, J.H.; Docherty, K.S.; Chhabra, P.S.; Bahreini, R.; et al. Organic aerosol components observed in Northern Hemispheric datasets from Aerosol Mass Spectrometry. Atmos. Chem. Phys. 2010, 10, 4625–4641.
- Cleveland, M.J.; Ziemba, L.D.; Griffin, R.J.; Dibb, J.E.; Anderson, C.H.; Lefer, B.; Rappenglück, B. Characterization of urban aerosol using aerosol mass spectrometry and proton nuclear magnetic resonance spectroscopy. Atmos. Environ. 2012, 54, 511–518.
- Shakya, K.M.; Place, P.F.; Griffin, R.J.; Talbot, R.W. Carbonaceous content and water-soluble organic functionality of atmospheric aerosols at a semi-rural New England location. J. Geophys. Res. Atmos. 2012, 117, 1–13.
- Timonen, H.; Carbone, S.; Aurela, M.; Saarnio, K.; Saarikoski, S.; Ng, N.L.; Canagaratna, M.R.; Kulmala, M.; Kerminen, V.-M.; Worsnop, D.R.; et al. Characteristics, sources and water-solubility of ambient submicron organic aerosol in springtime in Helsinki, Finland. J. Aerosol Sci. 2013, 56, 61–77.
- Chalbot, M.-C.G.; Brown, J.; Chitranshi, P.; Gamboa da Costa, G.; Pollock, E.D.; Kavouras, I.G. Functional characterization of the water-soluble organic carbon of size-fractionated aerosol in the southern Mississippi Valley. Atmos. Chem. Phys. 2014, 14, 6075–6088.
- Willoughby, A.S.; Wozniak, A.S.; Hatcher, P.G. A molecular-level approach for characterizing water-insoluble components of ambient organic aerosol particulates using ultrahigh-resolution mass spectrometry. Atmos. Chem. Phys. 2014, 14, 10299–10314.
- Nozière, B.; Kalberer, M.; Claeys, M.; Allan, J.; D’Anna, B.; Decesari, S.; Finessi, E.; Glasius, M.; Grgić, I.; Hamilton, J.F.; et al. The molecular identification of organic compounds in the atmosphere: State of the art and challenges. Chem. Rev. 2015, 115, 3919–3983.
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74.
- Basagaña, X.; Esnaola, M.; Rivas, I.; Amato, F.; Alvarez-Pedrerol, M.; Forns, J.; López-Vicente, M.; Pujol, J.; Nieuwenhuijsen, M.; Querol, X.; et al. Neurodevelopmental Deceleration by Urban Fine Particles from Different Emission Sources: A Longitudinal Observational Study. Environ. Health Perspect. 2016, 124, 1630–1636.
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the Air: A Review of the Effects of Particulate Matter Air Pollution on Human Health. J. Med. Toxicol. 2012, 8, 166–175.
- Shiraiwa, M.; Ueda, K.; Pozzer, A.; Lammel, G.; Kampf, C.J.; Fushimi, A.; Enami, S.; Arangio, A.M.; Fröhlich-Nowoisky, J.; Fujitani, Y.; et al. Aerosol Health Effects from Molecular to Global Scales. Environ. Sci. Technol. 2017, 51, 13545–13567.
- Cassee, F.R.; Héroux, M.-E.; Gerlofs-Nijland, M.E.; Kelly, F.J. Particulate matter beyond mass: Recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal. Toxicol. 2013, 25, 802–812.
- Riediker, M.; Zink, D.; Kreyling, W.; Oberdörster, G.; Elder, A.; Graham, U.; Lynch, I.; Duschl, A.; Ichihara, G.; Ichihara, S.; et al. Particle toxicology and health—where are we? Part. Fibre Toxicol. 2019, 16, 19.
- Saffari, A.; Daher, N.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Global Perspective on the Oxidative Potential of Airborne Particulate Matter: A Synthesis of Research Findings. Environ. Sci. Technol. 2014, 48, 7576–7583.
- Rao, L.; Zhang, L.; Wang, X.; Xie, T.; Zhou, S.; Lu, S.; Liu, X.; Lu, H.; Xiao, K.; Wang, W.; et al. Oxidative potential induced by ambient particulate matters with acellular assays: A review. Processes 2020, 8, 1410.
- Akhtar, U.S.; Rastogi, N.; McWhinney, R.D.; Urch, B.; Chow, C.W.; Evans, G.J.; Scott, J.A. The combined effects of physicochemical properties of size-fractionated ambient particulate matter on in vitro toxicity in human A549 lung epithelial cells. Toxicol. Rep. 2014, 1, 145–156.
- MohseniBandpi, A.; Eslami, A.; Shahsavani, A.; Khodagholi, F.; Alinejad, A. Physicochemical characterization of ambient PM2.5 in Tehran air and its potential cytotoxicity in human lung epithelial cells (A549). Sci. Total Environ. 2017, 593, 182–190.
- Park, M.; Joo, H.S.; Lee, K.; Jang, M.; Kim, S.D.; Kim, I.; Borlaza, L.J.S.; Lim, H.; Shin, H.; Chung, K.H.; et al. Differential toxicities of fine particulate matters from various sources. Sci. Rep. 2018, 8, 1–11.
- Xu, F.; Qiu, X.; Hu, X.; Shang, Y.; Pardo, M.; Fang, Y.; Wang, J.; Rudich, Y.; Zhu, T. Effects on IL-1β signaling activation induced by water and organic extracts of fine particulate matter (PM2.5) in vitro. Environ. Pollut. 2018, 237, 592–600.
- Molina, C.; Toro, A.R.; Manzano, C.A.; Canepari, S.; Massimi, L.; Leiva-Guzmán, M.A. Airborne aerosols and human health: Leapfrogging from mass concentration to oxidative potential. Atmosphere 2020, 11, 917.
- Lin, P.; Yu, J.Z. Generation of reactive oxygen species mediated by Humic-like substances in atmospheric aerosols. Environ. Sci. Technol. 2011, 45, 10362–10368.
- Verma, V.; Rico-Martinez, R.; Kotra, N.; King, L.; Liu, J.; Snell, T.W.; Weber, R.J. Contribution of water-soluble and insoluble components and their hydrophobic/hydrophilic subfractions to the reactive oxygen species-generating potential of fine ambient aerosols. Environ. Sci. Technol. 2012, 46, 11384–11392.
- Daher, N.; Ruprecht, A.; Invernizzi, G.; De Marco, C.; Miller-Schulze, J.; Heo, J.B.; Shafer, M.M.; Shelton, B.R.; Schauer, J.J.; Sioutas, C. Characterization, sources and redox activity of fine and coarse particulate matter in Milan, Italy. Atmos. Environ. 2012, 49, 130–141.
- Saffari, A.; Daher, N.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Seasonal and spatial variation in reactive oxygen species activity of quasi-ultrafine particles (PM0.25) in the Los Angeles metropolitan area and its association with chemical composition. Atmos. Environ. 2013, 79, 566–575.
- Fang, T.; Verma, V.; Bates, J.T.; Abrams, J.; Klein, M.; Strickland, J.M.; Sarnat, E.S.; Chang, H.H.; Mulholland, A.J.; Tolbert, E.P.; et al. Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: Contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos. Chem. Phys. 2016, 16, 3865–3879.
- Velali, E.; Papachristou, E.; Pantazaki, A.; Choli-Papadopoulou, T.; Planou, S.; Kouras, A.; Manoli, E.; Besis, A.; Voutsa, D.; Samara, C. Redox activity and in vitro bioactivity of the water-soluble fraction of urban particulate matter in relation to particle size and chemical composition. Environ. Pollut. 2016, 208, 774–786.
- Visentin, M.; Pagnoni, A.; Sarti, E.; Pietrogrande, M.C. Urban PM2.5 oxidative potential: Importance of chemical species and comparison of two spectrophotometric cell-free assays. Environ. Pollut. 2016, 219, 72–79.
- Wang, X.; Qin, Y.; Qin, J.; Yang, Y.; Qi, T.; Chen, R.; Tan, J.; Xiao, K. The interaction laws of atmospheric heavy metal ions and water-soluble organic compounds in PM2.5 based on the excitation-emission matrix fluorescence spectroscopy. J. Hazard. Mater. 2021, 402, 123497.
- Moonshine, M.; Rudich, Y.; Katsman, S.; Graber, E.R. Atmospheric HULIS enhance pollutant degradation by promoting the dark Fenton reaction. Geophys. Res. Lett. 2008, 35, 2–5.
- Russell, L.M. Aerosol Organic-Mass-to-Organic-Carbon Ratio Measurements. Environ. Sci. Technol. 2003, 37, 2982–2987.
- Na, K.; Cocker, D.R. Organic and elemental carbon concentrations in fine particulate matter in residences, schoolrooms, and outdoor air in Mira Loma, California. Atmos. Environ. 2005, 39, 3325–3333.
- Rivas, I.; Viana, M.; Moreno, T.; Bouso, L.; Pandolfi, M.; Alvarez-Pedrerol, M.; Forns, J.; Alastuey, A.; Sunyer, J.; Querol, X. Outdoor infiltration and indoor contribution of UFP and BC, OC, secondary inorganic ions and metals in PM2.5 in schools. Atmos. Environ. 2015, 106, 129–138.
- Farmer, D.K.; Vance, M.E.; Abbatt, J.P.D.; Abeleira, A.; Alves, M.R.; Arata, C.; Boedicker, E.; Bourne, S.; Cardoso-Saldaña, F.; Corsi, R.; et al. Overview of HOMEChem: House Observations of Microbial and Environmental Chemistry. Environ. Sci. Process. Impacts 2019, 21, 1280–1300.
- Abbatt, J.P.D.; Wang, C. The atmospheric chemistry of indoor environments. Environ. Sci. Process. Impacts 2020, 22, 25–48.
- Bekö, G.; Carslaw, N.; Fauser, P.; Kauneliene, V.; Nehr, S.; Phillips, G.; Saraga, D.; Schoemaecker, C.; Wierzbicka, A.; Querol, X. The past, present, and future of indoor air chemistry. Indoor Air 2020, 30, 373–376.
- Lai, A.M.; Carter, E.; Shan, M.; Ni, K.; Clark, S.; Ezzati, M.; Wiedinmyer, C.; Yang, X.; Baumgartner, J.; Schauer, J.J. Chemical composition and source apportionment of ambient, household, and personal exposures to PM 2.5 in communities using biomass stoves in rural China. Sci. Total Environ. 2019, 646, 309–319.
- Hasheminassab, S.; Daher, N.; Shafer, M.M.; Schauer, J.J.; Delfino, R.J.; Sioutas, C. Chemical characterization and source apportionment of indoor and outdoor fine particulate matter (PM2.5) in retirement communities of the Los Angeles Basin. Sci. Total Environ. 2014, 490, 528–537.
- Huang, W.; Baumgartner, J.; Zhang, Y.; Wang, Y.; Schauer, J.J. Source apportionment of air pollution exposures of rural Chinese women cooking with biomass fuels. Atmos. Environ. 2015, 104, 79–87.
- Arhami, M.; Minguillón, M.C.; Polidori, A.; Schauer, J.J.; Delfino, R.J.; Sioutas, C. Organic compound characterization and source apportionment of indoor and outdoor quasi-ultrafine particulate matter in retirement homes of the Los Angeles Basin. Indoor Air 2010, 20, 17–30.
- Li, J.; Xu, W.; Li, Z.; Duan, M.; Ouyang, B.; Zhou, S.; Lei, L.; He, Y.; Sun, J.; Wang, Z.; et al. Real-time characterization of aerosol particle composition, sources and influences of increased ventilation and humidity in an office. Indoor Air 2021, 31, 1364–1376.
- Cummings, B.E.; Waring, M.S. Predicting the importance of oxidative aging on indoor organic aerosol concentrations using the two-dimensional volatility basis set (2D-VBS). Indoor Air 2019, 29, 616–629.
- Jimenez, J.L.; Canagaratna, M.R.; Donahue, N.M.; Prevot, A.S.H.; Zhang, Q.; Kroll, J.H.; DeCarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N.L.; et al. Evolution of Organic Aerosols in the Atmosphere. Science 2009, 326, 1525–1529.
- Duncan, S.M.; Sexton, K.; Collins, L.; Turpin, B.J. Residential water-soluble organic gases: Chemical characterization of a substantial contributor to indoor exposures. Environ. Sci. Process. Impacts 2019, 21, 1364–1373.
- Duncan, S.M.; Tomaz, S.; Morrison, G.; Webb, M.; Atkin, J.; Surratt, J.D.; Turpin, B.J. Dynamics of Residential Water-Soluble Organic Gases: Insights into Sources and Sinks. Environ. Sci. Technol. 2019, 53, 1812–1821.
