Hazelnut is one of the four major nuts in the world and has high nutritional and economic value. This study employed Illumina sequencing of ITS rDNA and 16S rRNA genes to identify the seasonal changes in soil microbial community, the predominant environmental factors driving microbial community composition, and the differences in soil microbial composition among different species of the genus Corylus. We found that the soil microbial community composition of species of Corylus changed significantly with the change in seasons. Corylus heterophylla and Corylus kweichowensis had more ectomycorrhiza in their soil compared to Corylus avellane. The main factor influencing fungal community composition in soil was the available potassium, while that of bacteria was the total phosphorus content.
1. Introduction
Hazelnut, produced by a shrub or small tree of
Corylus Linn. in the family Betulaceae, is one of the four major dried fruits in the world and was reported to have originated in southwest China in the middle Eocene (~43.6 million years ago)
[1][2]. Among Chinese hazel plants,
Corylus heterophylla, mainly distributed in northern China, has been developed and utilized, while
Corylus kweichowensis, predominantly distributed in southern China, has important potential utilization value
[3].
Corylus avellane was introduced in China at the end of the 19th century, and a new hybrid hazel germplasm named Ping’ou which was hybridized by
C. heterophylla and
C. avellane had the advantages of strong resistance, high yield, and large fruit was obtained in the 1980s
[4][5].
Soil biological properties and soil microbial composition change with the seasons
[6][7], potentially in relation to seasonal differences in soil temperature, moisture, and soil organic matter content or autecological dynamics
[8][9][10]. Seasonal environmental variables, photosynthesis, root exudates, and litter can significantly change the composition of soil microbial communities
[11][12]. Previous studies showed that as the seasons changed, there was no significant change in β-glucosidase, urease, and acid phosphatase activities in the soil of a hazelnut orchard
[13]. However, seasonal changes in the soil microbial community structure of
Corylus have not yet been elucidated, and the environmental factors driving changes in the soil microbial community of
Corylus have not been studied to date. Clarifying the seasonal dynamics of the soil microbial community of
Corylus will facilitate the understanding of the composition of the soil microbial community of
Corylus.
Vegetation type is the predominant factor influencing the construction of soil microbial communities
[14][15][16]. The microbial community in topsoil is directly affected by vegetation types, because the difference in decomposability of litter produced by different tree species affects the abundance of microorganisms
[10]. Root exudates of different types of plants may also affect the composition of soil microbial communities in deep soil
[17]. Furthermore, vegetation types may indirectly affect microbial composition by regulating soil physical and chemical properties, which can directly affect soil microbial community composition
[18][19]. However, there are few studies on the seasonal differences in soil microorganisms of vegetation that are of the same genus, but different species.
Soil microorganisms usually form a complex interspecific network
[20]. Co-occurrence network analysis is an effective method to explore the interactions between different entities in the system and has been used to study various complex ecosystems
[21][22]. However, research on the seasonal changes of co-occurrence network analysis among species of the genus
Corylus is limited; such information could reveal the differences in the microbial community network among different species of the genus
Corylus and the influence of seasonal changes on the network.
2. Analysis on Results
2.1. Soil Physicochemical Properties
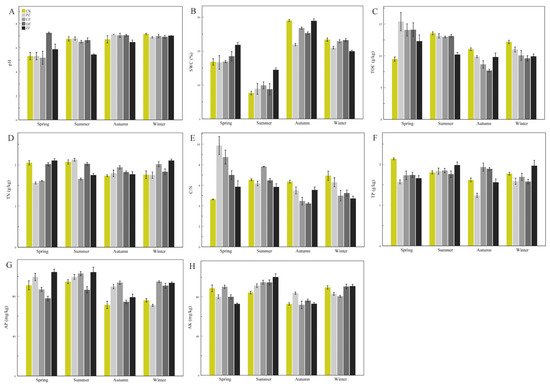
The soil physicochemical properties of the four species of the genus
Corylus in the four seasons are depicted in
Figure 1. Soil pH ranged from 5.17 (CZspr) to 7.23 (OZspr) (
Figure 1A,
Table S1), and the pH for
C.
heterophylla (PZ) in the spring was significantly different from that of the other three seasons (
Table S2). There were marked differences in pH between seasons for
C.
kweichowensis (CZ) except for autumn and winter. The pH for
C.
avellane (OZ) was significantly different in the summer compared with the other three seasons, and there were significant differences among the four seasons for
C.
heterophylla ×
C.
avellane (ZJ) (
p < 0.05,
Table S2). There was no significant difference in soil pH between the four species of
Corylus in the winter, and all species except OZ (pH 7.23) had a lower pH in the spring (
Table S2). SWC for the four species of
Corylus showed significant differences in each season (
p < 0.05,
Table S2). SWC for all four species was lowest in the summer and showed a trend of decreasing, then increasing, and then decreasing again (
Figure 1B). TOC displayed a similar trend of decreasing first and then increasing, and it reached the minimum value in autumn (
Figure 1C). TN values of CZ and OZ were not significantly different between spring and summer, while PZ and ZJ exhibited the opposite (
p < 0.05,
Table S2). There was also no significant difference between autumn and winter for CZ, OZ, and PZ (
Figure 1D,
Table S2). Except for ZJ, the value for the C/N ratio decreased between spring and autumn, and then increased in winter (
Figure 1E). Soil TP values of CZ and OZ increased initially between spring and autumn and then decreased in winter, while the values of PZ and ZJ tended to increase between spring and summer, then decreased in autumn, and increased again in winter (
Figure 1F). The AP values of CZ and OZ increased between spring and summer, decreased in autumn, and then increased again in winter, while the AP values of PZ showed a continual decrease through the seasons from spring to winter, and those of ZJ decreased from summer to autumn, and then increased in winter (
Figure 1G). The AK values of the four species increased initially between spring and summer, then decreased in autumn, before increasing again in winter (
Figure 1H). There were significant differences in AK values between CZ and OZ in the four seasons (
Table S2).
Figure 1. Soil physicochemical properties of four species of the genus Corylus across different seasons. (A) pH; (B) SWC; (C) TOC content; (D) TN content; (E) C/N content; (F) TP content; (G) AP content; (H) AK content. SWC: soil water content; TOC: total organic carbon; TN: total nitrogen; C/N: ratio of C and N; TP: total phosphorus; AP: available phosphorus; AK: available potassium; CK: control; PZ: C. heterophylla; CZ: C. kweichowensis; OZ: C. avellane; ZJ: C. heterophylla × C. avellane.
2.2. Soil Microbial Diversity
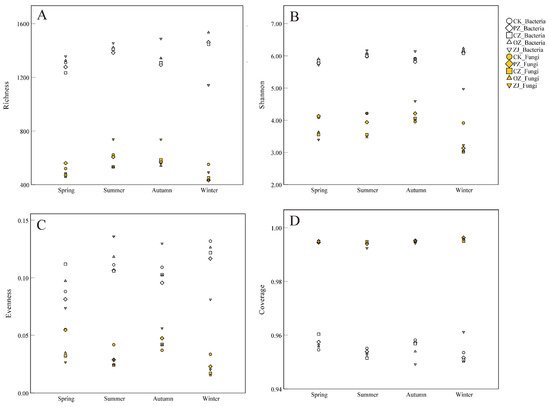
As shown in
Figure 2, the fungal OTU richness index ranged from 432.7 (PZwin) to 738.3 (ZJsum), while the bacterial OTU richness index ranged from 1141.3 (ZJwin) to 1533.3 (OZwin) (
Table S3). The OTU richness index of fungi in PZ soil in the winter was significantly different from that of the other three seasons (
p < 0.05), and the OTU richness index of fungi in CZ soil in winter was significantly different from that in autumn (
p < 0.05). There were significant differences between ZJspr and ZJwin, as well as ZJsum and ZJaut (
p < 0.05). Except for the significant differences between ZJsum, ZJaut, and the other two seasons, there were no significant differences in the fungal OTU richness index among different species of
Corylus in the same season. In bacteria, except ZJwin, there were no significant differences in OTU richness index among different species of
Corylus in the same season (
Table S3). The seasonal variation trend in the OTU richness index of fungi was an initial increase between spring and autumn, followed by a decrease in winter, while for bacteria, the trend in PZ, CZ and OZ was an initial increase between spring and summer, then a decrease in autumn, and an increase again in winter. In contrast, ZJ showed an increase in bacterial OTU richness index between spring and autumn before decreasing in winter. Significant differences and trends in the Shannon index of bacteria among samples were similar to those of the OTU richness index. Bacterial evenness indices of CK, PZ, CZ, and OZ showed no significant differences in each season, but there was an obvious difference between summer and autumn for ZJ. The bacterial evenness index trend of CZ was an initial decrease between spring and autumn followed by an increase in winter. In contrast, ZJ displayed an initial increase between spring and summer and then decreased, and the other three samples (CK, PZ, and OZ) increased between spring and summer, decreased in autumn, and then increased again in winter. The whole seasonal variation trend of evenness index of fungi was opposite to that of bacteria. All samples had good coverage, and the average value was above 0.95 (Fungi: 0.99; Bacteria: 0.95). The richness index, Shannon index, and evenness index values of bacteria were higher than those of fungi.
Figure 2. Diversity indices of soil microbial communities. (A) OTU richness index; (B) Shannon index; (C) Community evenness; (D) Community coverage. CK: control; PZ: C. heterophylla; CZ: C. kweichowensis; OZ: C. avellane; ZJ: C. heterophylla × C. avellane.
2.3. Soil Microbial Community Structure and Function
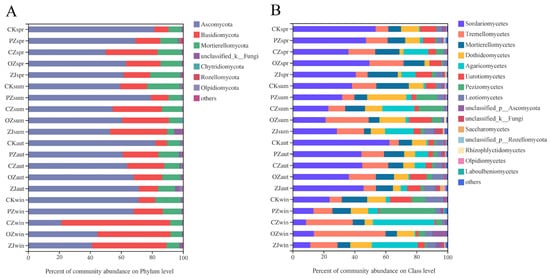
The predominant fungi phyla in all samples were Ascomycota (66.21%), Basidiomycota (22.82%), and Mortierellomycota (9.32%) (Figure 3A). Seasonal variation of Ascomycota content occurred in OZ and ZJ soils with an initial decrease between spring and summer, followed by an increase in autumn, and then a decrease in winter, while PZ showed the opposite trend, and CZ increased between spring and autumn and then markedly decreased in winter. The seasonal variation trend of Basidiomycota and Mortierellomycota contents in PZ, OZ, and ZJ soils were consistent with the variation trend of Ascomycota content in OZ soil. At the class level of fungi, the main classes in all samples were Sordariomycetes (34.74%), Tremellomycetes (15.62%), and Mortierellomycetes (11.70%) (Figure 3B). Seasonal variation of Sordariomycetes in all samples comprised an initial decrease from spring to summer, then an increase in autumn, followed by a decrease in winter. Seasonal variation of Tremellomycetes content in PZ soil was consistent with that of Sordariomycetes content, but contrary to the seasonal variation of Tremellomycetes content observed for OZ and ZJ. Tremellomycetes content in CZ soil decreased between spring and summer and then increased from autumn onwards. Seasonal variation of Mortierellomycetes content in each sample was consistent with that of the phylum Mortierellomycota.
Figure 3. Relative abundances of soil fungal community structure at phylum (A) and class (B) levels. CK: control; PZ: C. heterophylla; CZ: C. kweichowensis; OZ: C. avellane; ZJ: C. heterophylla × C. avellane; spr: spring; sum: summer; aut: autumn; win: winter.
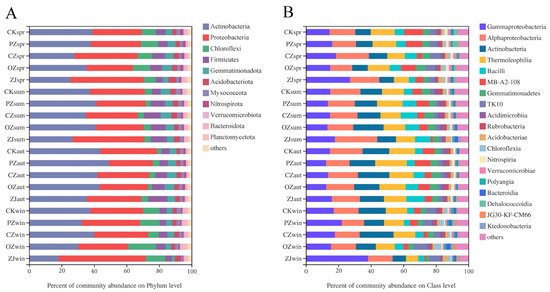
At the bacterial phylum level, the dominant taxa in all samples were Actinobacteria (36.08%) and Proteobacteria (34.22%) (Figure 4A). The seasonal variation trend of Actinobacteria content of all four species of Corylus was an initial increase between spring and autumn, followed by a decrease in winter. The seasonal variation trend of Proteobacteria content in PZ, CZ, and ZJ soils was an initial decrease between spring and autumn, followed by an increase in winter, while OZ showed a continuous increase throughout the seasons from spring to winter. At the bacterial class level, the dominant taxa in all samples were Gammaproteobacteria (17.39%), Alphaproteobacteria (16.95%), Actinobacteria (13.40%), and Thermoleophilia (13.28%) (Figure 4B). Gammaproteobacteria content in the soil of all four species of Corylus initially decreased between spring and autumn, and then increased in winter. Seasonal variation trends of the content of the class Alphaproteobacteria in PZ, OZ, and ZJ samples were consistent with those of Gammaproteobacteria. The seasonal variation trend of Actinobacteria content in CZ soil is a continuous increase, reaching the maximum in winter, while PZ, OZ and ZJ all increased initially and then decreased, reaching the maximum in summer (OZ) and autumn (PZ, ZJ), respectively.
Figure 4. Relative abundances of soil bacterial community structure at phylum (A) and class (B) levels. CK: control; PZ: C. heterophylla; CZ: C. kweichowensis; OZ: C. avellane; ZJ: C. heterophylla × C. avellane; spr: spring; sum: summer; aut: autumn; win: winter.
There were significant differences (
p < 0.05) in the class level (the first 15 classes) of soil fungi of all four species of the genus
Corylus in the spring, summer, and winter, while for bacteria, significant differences were observed at the class level for all four species of
Corylus in all seasons except spring (
Figures S2 and S3). In spring, the class of soil fungi with significantly different abundance between the four species of
Corylus was
Saccharomycetes, while in summer, the significantly different fungal classes were
Sordariomycetes,
Agaricomycetes,
unclassified_p__Ascomycota,
Saccharomycetes, and
Zoopagomycetes, and in winter, they were
Agaricomycetes,
Eurotiomycetes,
Taphrinomycetes, and
Microbotryomycetes. Among the bacteria,
MB-A2-108,
Rubobacter, Acidobacteriae,
Verrucomicrobiae, and
Holophagae were the classes with significantly different abundances in soil between the species of
Corylus in summer. In autumn, the significantly different classes were
Bacilli and
Rubrobacteria, and in winter,
Gammaproteobacteria was the only class displaying a significant difference in abundance among soil samples of the species of
Corylus.
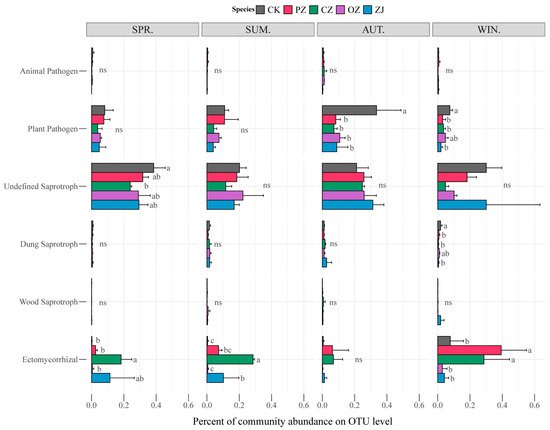
The seasonal changes of soil fungal function for each species of the genus
Corylus sampled in this study are shown in
Figure 5. The functions of animal pathogen and plant pathogen belong to pathotroph; undefined saprotroph, dung saprotroph, and wood saprotroph belong to saprotroph; and ectomycorrhizal belongs to symbiotroph. The pathotroph guild was dominated by the function of plant pathogen, while saprotroph was dominated by undefined saprotroph. Statistical analysis of the guilds revealed that there were no significant differences among the four species of
Corylus in each season except for the ectomycorrhizal guild, which showed an obvious change in abundance with the seasons. In spring and summer, the ectomycorrhizal abundance of CZ was significantly higher than that of other species of the genus
Corylus, and in winter, the ectomycorrhizal abundance of PZ and CZ was significantly higher than that of OZ and ZJ. According to the prediction results of bacterial function by PICRUSt2, there were no significant differences among the four species of the genus
Corylus examined in this study (
Figure S4).
Figure 5. Functional features of fungal communities in four species of the genus Corylus in different seasons. CK: control; PZ: C. heterophylla; CZ: C. kweichowensis; OZ: C. avellane; ZJ: C. heterophylla × C. avellane; spr: spring; sum: summer; aut: autumn; win: winter. Different letters (a,b) indicate the significance level at p < 0.05, ns indicate no significance (p > 0.05).
2.4. Relationships of Microbial Communities and Soil Properties
AP was the major driving factor of soil fungal community in PZ (
p < 0.05), while pH, TOC, and TN were the predominant driving factors of bacterial community composition in CZ (
p < 0.05). TP and AP had significant effects on both fungal and bacterial communities in OZ (
p < 0.05). In addition, pH and AK were also the main environmental drivers of fungal communities in OZ (
p < 0.05). TP and AK were the main environmental drivers of fungal community composition in ZJ (
p < 0.05), while pH and TN had significant effects on bacterial community composition in ZJ (
p < 0.05) (
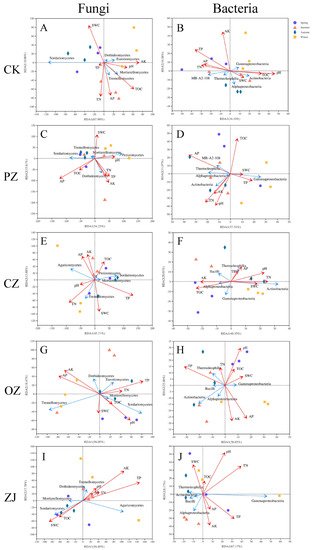
Table S4). In soil microorganisms, there were also strong correlations among environmental factors. For example, in soil fungi of PZ and ZJ, there was a strong correlation between AP and TN, and in soil bacteria of OZ and ZJ, there was a strong correlation between pH, TOC, and SWC (
Figure 6). Correlation analysis between environmental factors and the first 20 classes of fungi and bacteria (
Figure 6 and
Figure S7) indicated that AK was the predominant environmental factor affecting fungal community composition and TP was the main one affecting bacterial community composition.
Figure 6. Redundancy analysis (RDA) of the top five fungal and bacterial classes with soil proper ties. RDA of top five fungal classes with soil properties of CK (A), PZ (B), CZ (C), OZ (D), and ZJ (E) samples. RDA of top five bacterial classes and soil properties of CK (F), PZ (G), CZ (H), OZ (I), and ZJ (J) samples. CK: control; PZ: C. heterophylla; CZ: C. kweichowensis; OZ: C. avellane; ZJ: C. heterophylla × C. avellane.
3. Current Insights
Seasonal changes can affect the diversity of microorganisms
[23][24][25][26], and in general, the richness of bacteria in the same habitat is higher than that of fungi. The diversity of bacteria in this study was significantly higher than that of fungi (
Figure 2), congruent with previous studies
[20][27]. There were significant differences in fungal diversity among seasons, but there was no significant difference in bacterial diversity among seasons (
Table S2). Bacteria have a wider range of life and often form biofilms in the soil
[28]. Therefore, fungi are more susceptible to precipitation and temperature changes caused by seasonal changes than bacterial communities; hence fungi and bacteria have different adaptability to environmental changes
[20][29]. The change trend of fungal Shannon diversity in the current study was similar to that of He et al.
[20], and the change trend of bacterial diversity was also similar to previous studies
[27][30]. The increase in soil moisture caused by summer precipitation may be one reason for the increase in microbial richness
[31][32]. The nutrient supply in autumn was related to the decrease in total bacterial community diversity during these periods, and this may be due to dry conditions and limited nutrient conditions that were previously observed to result in decreased diversity in October
[33][34]. A previous study showed that seasonal changes in photosynthesis had a greater impact on soil respiration compared with seasonal changes in soil temperature, and that a decrease in soil respiration and soil temperature leads to a decrease in microbial diversity
[35]. However, in the current study, bacterial diversity did not decrease in the winter. This may be due to the input of litter, which increases organic matter in the soil
[36][37]. In addition, after entering autumn, precipitation markedly decreased in the present study, and because fungi are more susceptible to drought stress than bacteria, the diversity of fungi will therefore decrease
[20][28]. Another possible reason for the increased bacterial diversity in the current study is that the soil in the area sampled was not very sensitive to the above factors, and another potential explanation is that plants have less demand for soil nutrients in winter, therefore bacterial diversity and abundance will increase due to the availability of nutrients for the bacteria.
The community composition of fungi and bacteria showed obvious seasonal changes (
Figure 3 and
Figure 4). Season was a key driving force of soil microorganisms, and this was in agreement with previous reports
[38]. According to reported studies, some members of Agaricomycetes are related to ectomycorrhiza. Ectomycorrhiza can promote the growth of trees
[39].
C.
heterophylla (PZ),
C.
kweichowensis (CZ), and
C.
heterophylla ×
C.
avellane (ZJ) all had a high proportion of
Agaricomycetes in each season. This was consistent with the functional abundance of symbiotic bacteria predicted by FUNGuild in
Figure 5. Sequencing results indicated that the main ectomycorrhizal genera of the class
Agaricomycetes were
Hymenogaster,
Scleroderma,
Hebeloma,
Tomentella, and
Tuber. PZ and CZ had more ectomycorrhizal symbionts than
C. avellane (OZ) had. Among them,
Tuber is a rare edible fungus with important nutritional and economic value
[40], including several species of truffles. Most truffles coexist with trees or shrubs. Previous studies on the mycorrhizal effect of truffle on
C. avellane seedlings showed that
C. avellane can coexist with truffle and that truffle can improve the rooting rate and root length of the hazel cuttings
[41][42][43]. Truffles mainly grow in southwest China; therefore, the prediction of suitable areas for culture of
C. kweichowensis based on climate forecasting data from 2041 to 2060 can not only identify potential suitable areas for truffles but can also provide a theoretical basis for the establishment of
C. kweichowensis-truffle cultivation gardens.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9112228