Nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S), three major gasotransmitters, are involved in pleiotropic biofunctions. Research on their roles in hypertension and kidney disease has greatly expanded recently. The developing kidney can be programmed by various adverse in utero conditions by so-called renal programming, giving rise to hypertension and kidney disease in adulthood.
- kidney disease
- gasotransmitter
- carbon monoxide
- hypertension
- developmental origins of health and disease (DOHaD)
- hydrogen sulfide
- asymmetric dimethylarginine
- heme oxygenase
- nitric oxide
1. Introduction
|
Physiological Level |
Toxic Level |
||
|---|---|---|---|
|
Gas |
Blood Concentration |
Exposure Limit |
IDLH |
|
Nitric oxide (NO) |
Low nM |
TMA 25 ppm |
100 ppm |
|
Carbon monoxide (CO) |
nM–μM |
TMA 35 ppm/C 200 ppm |
100 ppm |
|
Hydrogen sulfide (H2S) |
High nM–low μM |
C 10 ppm |
1200 ppm |
2. Implications of Gasotransmitters in Pregnancy
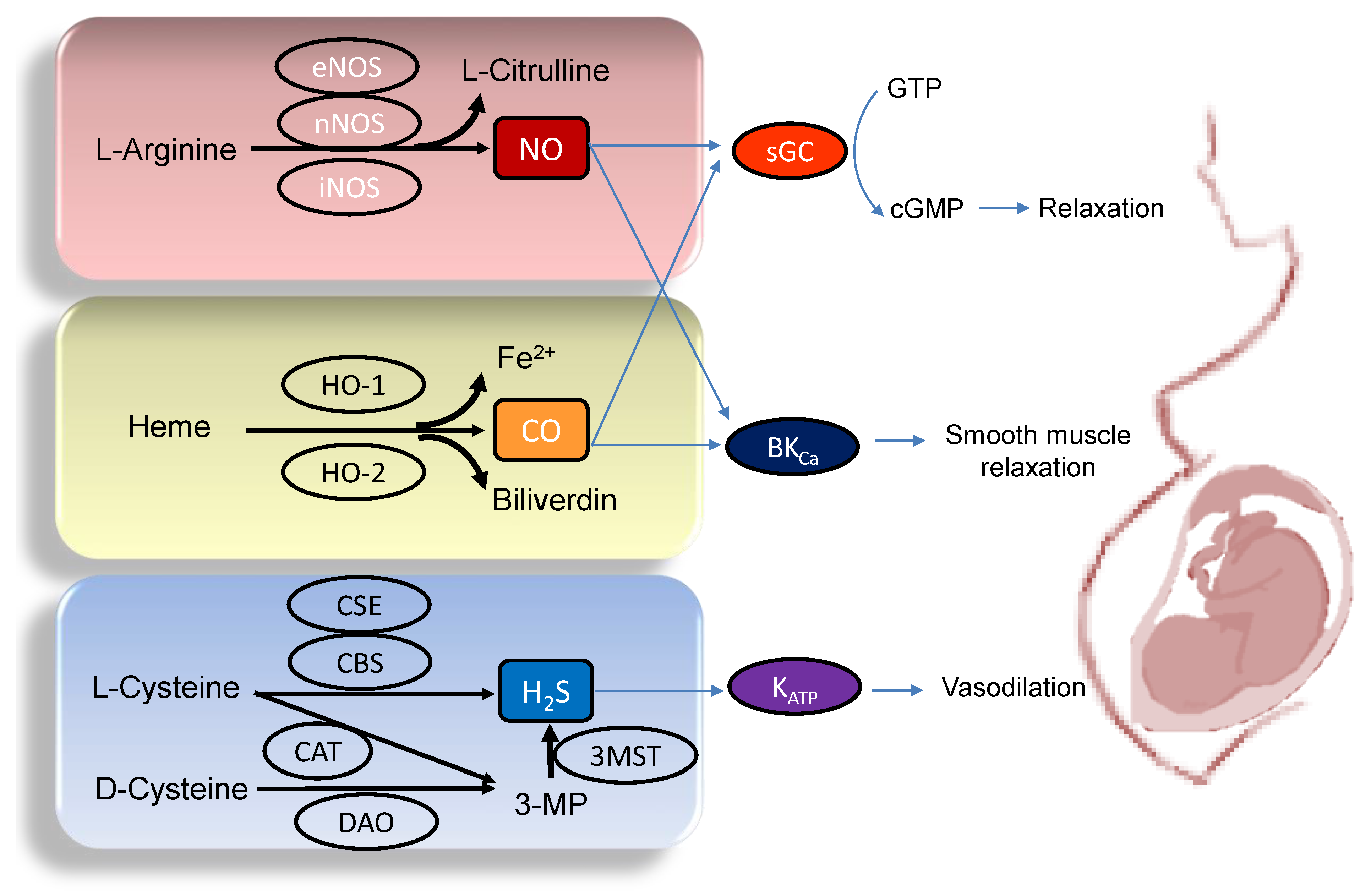
3. Implications of Gasotransmitters in Hypertension and Kidney Disease
3.1. Gasotransmitters and Hypertension
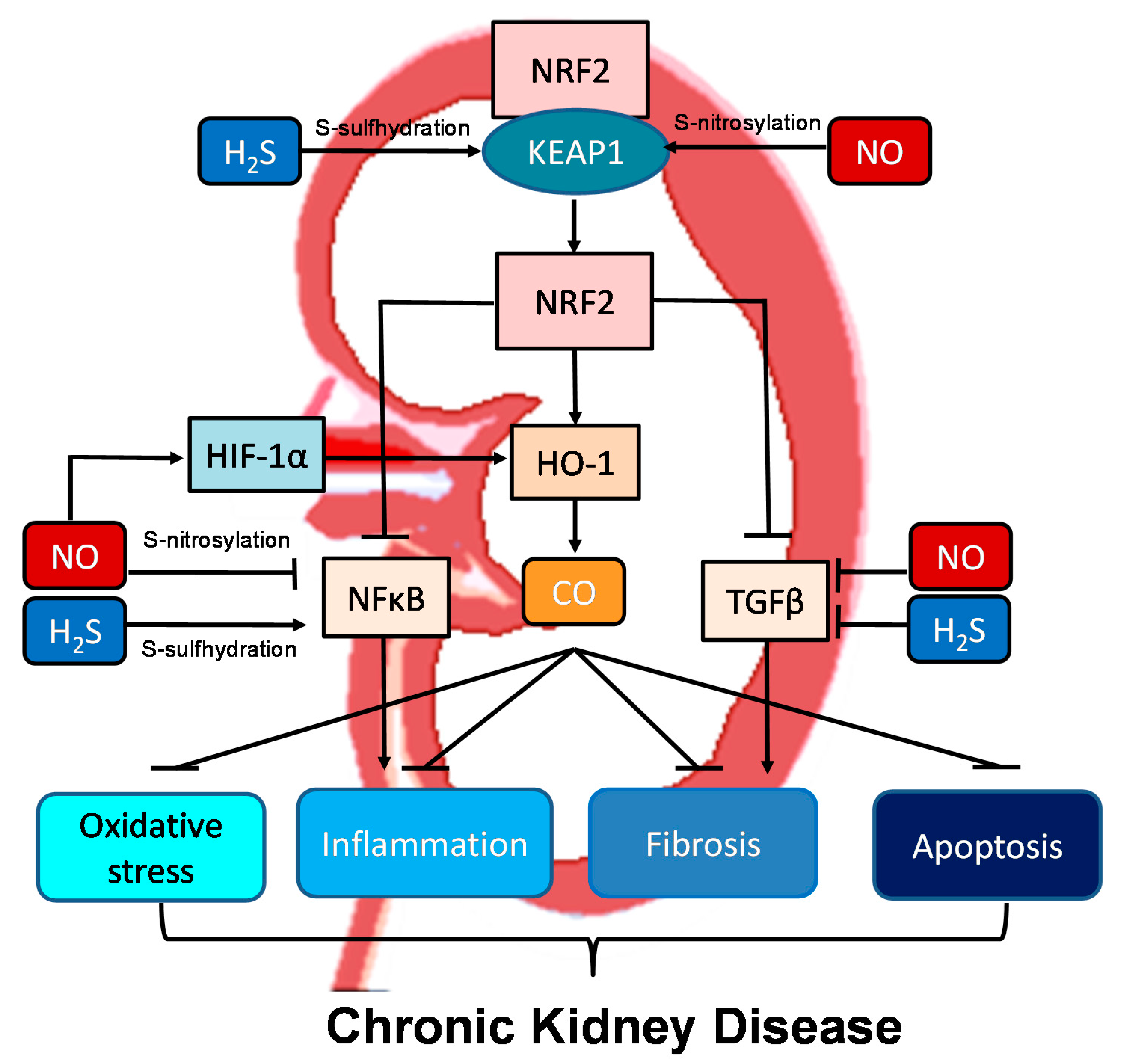
3.2. Gasotransmitters and Kidney Disease
3.3. Crosstalk between NO, CO, and H2S in the Kidney and BP Control
This entry is adapted from the peer-reviewed paper 10.3390/ijms22157808
References
- Kajimura, M.; Fukuda, R.; Bateman, R.M.; Yamamoto, T.; Suematsu, M. Interactions of multiple gas-transducing systems: Hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid. Redox Signal. 2010, 13, 157–192.
- Polhemus, D.J.; Lefer, D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014, 114, 730–737.
- NIOSH Pocket Guide to Chemical Hazards. 2020. Available online: https://www.cdc.gov/niosh/npg/ (accessed on 19 July 2021).
- Lancaster, J.R. Historical origins of the discovery of mammalian nitric oxide (nitrogen monoxide) production/physiology/pathophysiology. Biochem. Pharmacol. 2020, 176, 113793.
- Durante, W. Carbon monoxide and bile pigments: Surprising mediators of vascular function. Vasc. Med. 2002, 7, 195–202.
- Kimura, H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide 2014, 41, 4–10.
- World Health Organization. Hypertension. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 12 May 2021).
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128.
- Weir, M.R. Hypertension and the kidney: Perspectives on the relationship of kidney disease and cardiovascular disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 2045–2050.
- Tain, Y.L.; Hsu, C.N. Developmental origins of chronic kidney disease: Should we focus on early life? Int. J. Mol. Sci. 2017, 18, 381.
- Haugen, A.C.; Schug, T.T.; Collman, G.; Heindel, J.J. Evolution of DOHaD: The impact of environmental health sciences. J. Dev. Orig. Health Dis. 2015, 6, 55–64.
- Tain, Y.L.; Joles, J.A. Reprogramming: A preventive strategy in hypertension focusing on the kidney. Int. J. Mol. Sci. 2016, 17, 23.
- Paauw, N.D.; van Rijn, B.B.; Lely, A.T.; Joles, J.A. Pregnancy as a critical window for blood pressure regulation in mother and child: Programming and reprogramming. Acta Physiol. 2017, 219, 241–259.
- Chong, E.; Yosypiv, I.V. Developmental programming of hypertension and kidney disease. Int. J. Nephrol. 2012, 2012, 760580.
- Paixão, A.D.; Alexander, B.T. How the kidney is impacted by the perinatal maternal environment to develop hypertension. Biol. Reprod. 2013, 89, 144.
- Hsu, C.N.; Tain, Y.L. Animal models for DOHaD research: Focus on hypertension of developmental origins. Biomedicines 2021, 9, 623.
- Holwerda, K.M.; Faas, M.M.; van Goor, H.; Lely, A.T. Gasotransmitters: A solution for the therapeutic dilemma in preeclampsia? Hypertension 2013, 62, 653–659.
- Guerra, D.D.; Hurt, K.J. Gasotransmitters in pregnancy: From conception to uterine involution. Biol. Reprod. 2019, 101, 4–25.
- Rengarajan, A.; Mauro, A.K.; Boeldt, D.S. Maternal disease and gasotransmitters. Nitric Oxide 2020, 96, 1–12.
- Huang, P.L.; Huang, Z.; Mashimo, H.; Bloch, K.D.; Moskowitz, M.A.; Bevan, J.A.; Fishman, M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 1995, 377, 239–242.
- Shibuya, N.; Kimura, H. Production of hydrogen sulfide from d-cysteine and its therapeutic potential. Front. Endocrinol. 2013, 4, 87.
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590.
- Dayal, S.; Bottiglieri, T.; Arning, E.; Maeda, N.; Malinow, M.R.; Sigmund, C.D.; Heistad, D.D.; Faraci, F.M.; Lentz, S.R. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine beta-synthase-deficient mice. Circ. Res. 2001, 88, 1203–1209.
- Mani, S.; Li, H.; Untereiner, A.; Wu, L.; Yang, G.; Austin, R.C.; Dickhout, J.G.; Lhoták, Š.; Meng, Q.H.; Wang, R. Decreased Endogenous Production of Hydrogen Sulfide Accelerates Atherosclerosis. Circulation 2013, 127, 2523–2534.
- Stout, J.M.; Gousset, M.U.; Drummond, H.A.; Gray, W., 3rd; Pruett, B.E.; Stec, D.E. Sex-specific effects of heme oxygenase-2 deficiency on renovascular hypertension. J. Am. Soc. Hypertens. 2013, 7, 328–335.
- Tain, Y.L.; Hsu, C.N. Targeting on asymmetric dimethylarginine-related nitric oxide-reactive oxygen species imbalance to reprogram the development of hypertension. Int. J. Mol. Sci. 2016, 17, 2020.
- Durante, W. Targeting heme oxygenase-1 in vascular disease. Curr. Drug Targets 2010, 11, 1504–1516.
- Jin, S.; Teng, X.; Xiao, L.; Xue, H.; Guo, Q.; Duan, X.; Chen, Y.; Wu, Y. Hydrogen sulfide ameliorated L-NAME-induced hypertensive heart disease by the Akt/eNOS/NO pathway. Exp. Biol. Med. (Maywood). 2017, 242, 1831–1841.
- Huang, P.; Chen, S.; Wang, Y.; Liu, J.; Yao, Q.; Huang, Y.; Li, H.; Zhu, M.; Wang, S.; Li, L.; et al. Down-regulated CBS/H2S pathway is involved in high-salt-induced hypertension in Dahl rats. Nitric Oxide 2015, 46, 192–203.
- Xiao, L.; Dong, J.-H.; Jing-Hui, D.; Xue, H.-M.; Guo, Q.; Teng, X.; Wu, Y.-M. Hydrogen sulfide improves endothelial dysfunction via downregulating BMP4/COX-2 pathway in rats with hypertension. Oxid. Med. Cell. Longev. 2016, 2016, 1–10.
- Van Goor, H.; Born, J.C.V.D.; Hillebrands, J.-L.; Joles, J.A. Hydrogen sulfide in hypertension. Curr. Opin. Nephrol. Hypertens. 2016, 25, 107–113.
- Paulo, M.; Costa, D.E.F.R.; Bonaventura, D.; Lunardi, C.N.; Bendhack, L.M. Nitric oxide donors as potential drugs for the treatment of vascular diseases due to endothelium dysfunction. Curr. Pharm. Des. 2020, 26, 3748–3759.
- Ndisang, J.F.; Tabien, H.E.; Wang, R. Carbon monoxide and hypertension. J. Hypertens. 2004, 22, 1057–1074.
- Wen, Y.-D.; Wang, H.; Zhu, Y.Z. The Drug developments of hydrogen sulfide on cardiovascular disease. Oxid. Med. Cell. Longev. 2018, 2018, 4010395.
- Beck, K.F.; Pfeilschifter, J. Gasotransmitter synthesis and signalling in the renal glomerulus. Implications for glomerular diseases. Cell. Signal. 2021, 77, 109823.
- Kone, B.C. Nitric oxide synthesis in the kidney: Isoforms, biosynthesis, and functions in health. Semin. Nephrol. 2004, 24, 299–315.
- Baylis, C. Nitric oxide synthase derangements and hypertension in kidney disease. Curr. Opin. Nephrol. Hypertens. 2012, 21, 1–6.
- Wilcox, C.S. Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R913–R935.
- Csongradi, E.; Juncos, L.A.; Drummond, H.A.; Vera, T.; Stec, D.E. Role of carbon monoxide in kidney function: Is a little carbon monoxide good for the kidney? Curr. Pharm. Biotechnol. 2012, 13, 819–826.
- Lever, J.M.; Boddu, R.; George, J.F.; Agarwal, A. Heme Oxygenase-1 in Kidney Health and Disease. Antioxid. Redox Signal. 2016, 25, 165–183.
- Ndisang, J.F.; Jadhav, A. Hemin therapy improves kidney function in male streptozotocin-induced diabetic rats: Role of the heme oxygenase/atrial natriuretic peptide/adiponectin axis. Endocrinology 2014, 155, 215–229.
- Mackern-Oberti, J.P.; Llanos, C.; Carreno, L.J.; Riquelme, S.A.; Jacobelli, S.H.; Anegon, I.; Kalergis, A.M. Carbon monoxide exposure improves immune function in lupus-prone mice. Immunology 2013, 140, 123–132.
- Mosley, K.; Wembridge, D.E.; Cattell, V.; Cook, H.T. Heme oxygenase is induced in nephrotoxic nephritis and hemin, a stimulator of heme oxygenase synthesis, ameliorates disease. Kidney Int. 1998, 53, 672–678.
- Ferenbach, D.A.; Ramdas, V.; Spencer, N.; Marson, L.; Anegon, I.; Hughes, J.; Kluth, D.C. Macrophages expressing heme oxygenase-1 improve renal function in ischemia/reperfusion injury. Mol. Ther. 2010, 18, 1706–1713.
- Kie, J.H.; Kapturczak, M.H.; Traylor, A.; Agarwal, A.; Hill-Kapturczak, N. Heme oxygenase-1 deficiency promotes epithelial-mesenchymal transition and renal fibrosis. J. Am. Soc. Nephrol. 2008, 19, 1681–1691.
- Zager, R.A.; Johnson, A.C.; Becker, K. Acute unilateral ischemic renal injury induces progressive renal inflammation, lipid accumulation, histone modification, and ‘‘end-stage’’ kidney disease. Am. J. Physiol. Renal Physiol. 2011, 301, F1334–F1345.
- Scammahorn, J.J.; Nguyen, I.T.N.; Bos, E.M.; Van Goor, H.; Joles, J.A. Fighting oxidative stress with sulfur: Hydrogen sulfide in the renal and cardiovascular systems. Antioxidants 2021, 10, 373.
- Dugbartey, G.J. The smell of renal protection against chronic kidney disease: Hydrogen sulfide offers a potential stinky remedy. Pharm. Rep. 2018, 70, 196–205.
- Chen, Y.; Jin, S.; Teng, X.; Hu, Z.; Zhang, Z.; Qiu, X.; Tian, D.; Wu, Y. Hydrogen sulfide attenuates LPS-induced acute kidney injury by inhibiting inflammation and oxidative stress. Oxid. Med. Cell. Longev. 2018, 2018, 6717212.
- Cao, X.; Zhang, W.; Moore, P.K.; Bian, J. Protective smell of hydrogen sulfide and polysulfide in cisplatin-induced nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 313.
- Lin, S.; Visram, F.; Liu, W.; Haig, A.; Jiang, J.; Mok, A.; Lian, D.; Wood, M.E.; Torregrossa, R.; Whiteman, M.; et al. GYY4137, a slow-releasing hydrogen sulfide donor, ameliorates renal damage associated with chronic obstructive uropathy. J. Urol. 2016, 196, 1778–1787.
- Xue, R.; Hao, D.D.; Sun, J.P.; Li, W.W.; Zhao, M.M.; Li, X.H.; Chen, Y.; Zhu, J.H.; Ding, Y.J.; Liu, J.; et al. Hydrogen sulfide treatment promotes glucose uptake by increasing insulin receptor sensitivity and ameliorates kidney lesions in type 2 diabetes. Antioxid. Redox Signal. 2013, 19, 5–23.
- Feliers, D.; Lee, H.J.; Kasinath, B.S. Hydrogen sulfide in renal physiology and disease. Antioxid. Redox Signal. 2016, 25, 720–731.
- Cirino, G.; Vellecco, V.; Bucci, M. Nitric oxide and hydrogen sulfide: The gasotransmitter paradigm of the vascular system. Br. J. Pharmacol. 2017, 174, 4021–4031.
- Zhong, G.; Chen, F.; Cheng, Y.; Tang, C.; Du, J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J. Hypertens. 2003, 21, 1879–1885.
- Zhou, H.L.; Zhang, R.; Anand, P.; Stomberski, C.T.; Qian, Z.; Hausladen, A.; Wang, L.; Rhee, E.P.; Parikh, S.M.; Karumanchi, S.A.; et al. Metabolic reprogramming by the S-nitroso-CoA reductase system protects against kidney injury. Nature 2019, 565, 96–100.
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72.
- Stec, D.E.; Drummond, H.A.; Vera, T. Role of carbon monoxide in blood pressure regulation. Hypertension 2008, 51, 597–604.
- Imai, T.; Morita, T.; Shindo, T.; Nagai, R.; Yazaki, Y.; Kurihara, H.; Suematsu, M.; Katayama, S. Vascular smooth muscle cell-directed overexpression of heme oxygenase-1 elevates blood pressure through attenuation of nitric oxide-induced vasodilation in mice. Circ. Res. 2001, 89, 55–62.
- Thorup, C.; Jones, C.L.; Gross, S.S.; Moore, L.C.; Goligorsky, M.S. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am. J. Physiol. 1999, 277, F882–F889.
- Uddin, M.J.; Kim, E.H.; Hannan, M.A.; Ha, H. Pharmacotherapy against oxidative stress in chronic kidney disease: Promising small molecule natural products targeting Nrf2-HO-1 signaling. Antioxidants 2021, 10, 258.
- Fernando, V.; Zheng, X.; Walia, Y.; Sharma, V.; Letson, J.; Furuta, S. S-Nitrosylation: An emerging paradigm of redox signaling. Antioxidants 2019, 8, 404.
- Iciek, M.; Kowalczyk-Pachel, D.; Bilska-Wilkosz, A.; Kwiecień, I.; Górny, M.; Włodek, L. S-sulfhydration as a cellular redox regulation. Biosci. Rep. 2015, 36, e00304.
