Primary IgA nephropathy (IgAN) is a leading cause of chronic kidney disease and kidney failure for which there is no disease-specific treatment. However, this could change, since novel therapeutic approaches are currently being assessed in clinical trials, including complement-targeting therapies. An improved understanding of the role of the lectin and the alternative pathway of complement in the pathophysiology of IgAN has led to the development of these treatment strategies. Recently, in a phase 2 trial, treatment with a blocking antibody against mannose-binding protein-associated serine protease 2 (MASP-2, a crucial enzyme of the lectin pathway) was suggested to have a potential benefit for IgAN. Now in a phase 3 study, this MASP-2 inhibitor for the treatment of IgAN could mark the start of a new era of complement therapeutics where common diseases can be treated with these drugs. The clinical development of complement inhibitors requires a better understanding by physicians of the biology of complement, the pathogenic role of complement in IgAN, and complement-targeted therapies.
- complement
- kidney
- nephrology
1. Introduction to the Complement System
2. The Complement System in IgA Nephropathy
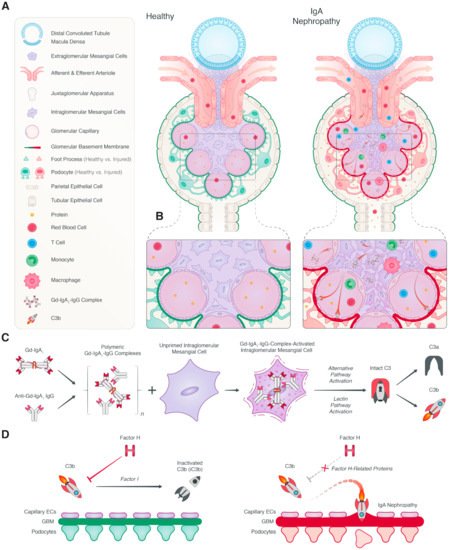
2.1. Local Complement Activation
| Evidence for the Involvement of the Factor H Protein Family in the Pathogenesis of IgA Nephropathy | |||
|---|---|---|---|
| Genetic | Histologic | Serologic | |
| Factor H | Genetic variants of Factor H associated with lower plasma levels may contribute to genetic susceptibility to IgAN [51]. | Glomerular deposition of Factor H staining is reduced in patients with progressive IgAN compared to stable disease. Absence of glomerular Factor H deposition is associated with progressive disease [39]. | Plasma Factor H levels are not altered in IgAN patients, and these levels are not associated with disease severity, but the plasma FHR-1/Factor H ratio is associated with disease progression [51][52]. |
| Factor H-related protein 1 (FHR-1) |
The deletion of complement factor H-related proteins 3 and 1 genes (CFHR3,1Δ) is associated with protection against IgAN [53][54][55][56]. | Proteomics showed that FHR-1 is more abundant in the glomeruli of IgAN patients compared to controls. Glomerular FHR-1 deposits have also been identified in IgAN, but no association is seen with IgAN severity [39][48]. | Plasma FHR-1 levels are elevated in IgAN patients compared to healthy controls, and the plasma FHR-1/Factor H ratio is associated with disease progression of the disease [51][52]. |
| Factor H-related protein 2 (FHR-2) |
N.D. | Proteomic analysis revealed that FHR-2 is more abundant in the glomeruli of patients with progressive IgAN compared to non-progressive IgAN [48]. | N.D. |
| Factor H-related protein 3 (FHR-3) |
The deletion of complement factor H-related proteins 3 and 1 genes (CFHR3,1Δ) is associated with protection against IgAN [53][54][55][56]. | Proteomic analysis demonstrated that FHR-3 is more abundant in the glomeruli of IgAN patients compared to controls [48]. | N.D. |
| Factor H-related protein 4 (FHR-4) |
N.D. | N.D. | N.D. |
| Factor H-related protein 5 (FHR-5) |
Rare genetic variants in FHR-5 may contribute to the genetic susceptibility to IgAN [57]. | Glomerular FHR-5 deposits have been identified in IgAN and correlate with C3 and C5b-9 deposits. Increased glomerular staining for FHR-5 is associated with more severe histology and progressive disease [39][48][49][50]. | Serum FHR-5 levels are higher in IgAN patients compared to healthy controls and are associated with more severe histology, unresponsiveness to immunosuppression, and disease progression [52][58]. |
2.2. Systemic Complement Activation
2.3. Genetic Variants in Complement Genes
This entry is adapted from the peer-reviewed paper 10.3390/jcm10204715
References
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797.
- Walport, M.J. Complement. First of two parts. N. Engl. J. Med. 2001, 344, 1058–1066.
- Walport, M.J. Complement. Second of two parts. N. Engl. J. Med. 2001, 344, 1140–1144.
- Ricklin, D.; Reis, E.S.; Lambris, J.D. Complement in disease: A defence system turning offensive. Nat. Rev. Nephrol. 2016, 12, 383–401.
- Noris, M.; Remuzzi, G. Overview of Complement Activation and Regulation. Semin. Nephrol. 2013, 33, 479–492.
- Garcia, B.L.; Zwarthoff, S.A.; Rooijakkers, S.H.M.; Geisbrecht, B.V. Novel Evasion Mechanisms of the Classical Complement Pathway. J. Immunol. 2016, 197, 2051–2060.
- Diebolder, C.; Beurskens, F.J.; de Jong, R.N.; Koning, R.; Strumane, K.; Lindorfer, M.A.; Voorhorst, M.; Ugurlar, D.; Rosati, S.; Heck, A.; et al. Complement Is Activated by IgG Hexamers Assembled at the Cell Surface. Science 2014, 343, 1260–1263.
- Da Costa, M.G.; Poppelaars, F.; Berger, S.P.; Daha, M.R.; Seelen, M.A. The lectin pathway in renal disease: Old concept and new insights. Nephrol. Dial. Transplant. 2018, 33, 2073–2079.
- Garred, P.; Genster, N.; Pilely, K.; Bayarri-Olmos, R.B.; Rosbjerg, A.; Ma, Y.J.; Skjoedt, M.O. A journey through the lectin pathway of complement-MBL and beyond. Immunol. Rev. 2016, 274, 74–97.
- Lachmann, P.J. The Amplification Loop of the Complement Pathways. In Advances in Immunology; Elsevier BV: Amsterdam, The Netherlands, 2009; Volume 104, pp. 115–149.
- Kemper, C.; Atkinson, J.P.; Hourcade, D.E. Properdin: Emerging Roles of a Pattern-Recognition Molecule. Annu. Rev. Immunol. 2010, 28, 131–155.
- O’Flynn, J.; Kotimaa, J.; Faber-Krol, R.; Koekkoek, K.; Klar-Mohamad, N.; Koudijs, A.; Schwaeble, W.J.; Stover, C.; Daha, M.R.; van Kooten, C. Properdin binds independent of complement activation in an in vivo model of anti-glomerular basement membrane disease. Kidney Int. 2018, 94, 1141–1150.
- Harboe, M.; Johnson, C.; Nymo, S.; Ekholt, K.; Schjalm, C.; Lindstad, J.K.; Pharo, A.; Hellerud, B.C.; Ekdahl, K.N.; Mollnes, T.E.; et al. Properdin binding to complement activating surfaces depends on initial C3b deposition. Proc. Natl. Acad. Sci. USA 2017, 114, e534–e539.
- Du Clos, T.W.; Mold, C. Pentraxins (CRP, SAP) in the process of complement activation and clearance of apoptotic bodies through Fcγ receptors. Curr. Opin. Organ Transplant. 2011, 16, 15–20.
- Inforzato, A.; Doni, A.; Barajon, I.; Leone, R.; Garlanda, C.; Bottazzi, B.; Mantovani, A. PTX3 as a paradigm for the interaction of pentraxins with the Complement system. Semin. Immunol. 2013, 25, 79–85.
- Roos, A.; Bouwman, L.H.; Van Gijlswijk-Janssen, D.J.; Faber-Krol, M.C.; Stahl, G.; Daha, M.R. Human IgA Activates the Complement System Via the Mannan-Binding Lectin Pathway. J. Immunol. 2001, 167, 2861–2868.
- Farrar, C.A.; Tran, D.; Li, K.; Wuding, Z.; Peng, Q.; Schwaeble, W.; Zhou, W.; Sacks, S.H. Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury. J. Clin. Investig. 2016, 126, 1911–1925.
- Bayly-Jones, C.; Bubeck, D.; Dunstone, M.A. The mystery behind membrane insertion: A review of the complement membrane attack complex. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160221.
- Ramm, L.E.; Whitlow, M.B.; Mayer, M.M. The relationship between channel size and the number of C9 molecules in the C5b-9 complex. J. Immunol. 1985, 134, 2594–2599.
- Zipfel, P.F.; Skerka, C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009, 9, 729–740.
- Wyatt, R.J.; Julian, B.A. IgA Nephropathy. N. Engl. J. Med. 2013, 368, 2402–2414.
- Suzuki, H.; Kiryluk, K.; Novak, J.; Moldoveanu, Z.; Herr, A.; Renfrow, M.B.; Wyatt, R.; Scolari, F.; Mestecky, J.; Gharavi, A.G.; et al. The Pathophysiology of IgA Nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1795–1803.
- Evans, D.J.; Williams, D.G.; Peters, D.K.; Sissons, J.G.P.; Boulton-Jones, J.M.; Ogg, C.S.; Cameron, J.S.; Hoffbrand, B.I. Glomerular Deposition of Properdin in Henoch-Schonlein Syndrome and Idiopathic Focal Nephritis. BMJ 1973, 3, 326–328.
- Maillard, N.; Wyatt, R.J.; Julian, B.A.; Kiryluk, K.; Gharavi, A.; Fremeaux-Bacchi, V.; Novak, J. Current Understanding of the Role of Complement in IgA Nephropathy. J. Am. Soc. Nephrol. 2015, 26, 1503–1512.
- Lang, Y.; Song, S.; Zhao, L.; Yang, Y.; Liu, T.; Shen, Y.; Wang, W. Serum IgA/C3 ratio and glomerular C3 staining predict progression of IgA nephropathy in children. Transl. Pediatr. 2021, 10, 666–672.
- Wu, D.; Li, X.; Yao, X.; Zhang, N.; Lei, L.; Zhang, H.; Tang, M.; Ni, J.; Ling, C.; Chen, Z.; et al. Mesangial C3 deposition and serum C3 levels predict renal outcome in IgA nephropathy. Clin. Exp. Nephrol. 2021, 25, 641–651.
- Kim, S.J.; Koo, H.M.; Lim, B.J.; Oh, H.J.; Yoo, D.E.; Shin, D.H.; Lee, M.J.; Doh, F.M.; Park, J.T.; Yoo, T.-H.; et al. Decreased Circulating C3 Levels and Mesangial C3 Deposition Predict Renal Outcome in Patients with IgA Nephropathy. PLoS ONE 2012, 7, e40495.
- Nam, K.H.; Joo, Y.S.; Lee, C.; Lee, S.; Kim, J.; Yun, H.-R.; Park, J.T.; Chang, T.I.; Ryu, D.-R.; Yoo, T.-H.; et al. Predictive value of mesangial C3 and C4d deposition in IgA nephropathy. Clin. Immunol. 2020, 211, 108331.
- Roos, A.; Rastaldi, M.P.; Calvaresi, N.; Oortwijn, B.D.; Schlagwein, N.; Van Gijlswijk-Janssen, D.J.; Stahl, G.; Matsushita, M.; Fujita, T.; van Kooten, C.; et al. Glomerular Activation of the Lectin Pathway of Complement in IgA Nephropathy Is Associated with More Severe Renal Disease. J. Am. Soc. Nephrol. 2006, 17, 1724–1734.
- Faria, B.; Canão, P.; Cai, Q.; Henriques, C.; Matos, A.C.; Poppelaars, F.; da Costa, M.G.; Daha, M.R.; Silva, R.; Pestana, M.; et al. Arteriolar C4d in IgA Nephropathy: A Cohort Study. Am. J. Kidney Dis. 2020, 76, 669–678.
- Espinosa, M.; Ortega, R.; Sánchez, M.; Segarra, A.; Salcedo, M.T.; González, F.; Camacho, R.; Valdivia, M.A.; Cabrera, R.; López, K.; et al. Association of C4d Deposition with Clinical Outcomes in IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2014, 9, 897–904.
- Segarra, A.; Romero, K.; Agraz, I.; Ramos, N.; Madrid, A.; Carnicer, C.; Jatem, E.; Vilalta, R.; Lara, L.E.; Ostos, E.; et al. Mesangial C4d Deposits in Early IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2017, 13, 258–264.
- McCoy, R.C.; Abramowsky, C.R.; Tisher, C.C. IgA nephropathy. Am. J. Pathol. 1974, 76, 123–144.
- Lee, H.-J.; Choi, S.Y.; Jeong, K.H.; Sung, J.-Y.; Moon, S.K.; Moon, J.-Y.; Lee, S.-H.; Lee, T.-W.; Ihm, C.-G. Association of C1q deposition with renal outcomes in IgA nephropathy. Clin. Nephrol. 2013, 80, 98–104.
- Rauterberg, E.W.; Lieberknecht, H.M.; Wingen, A.M.; Ritz, E. Complement membrane attack (MAC) in idiopathic IgA-glomerulonephritis. Kidney Int. 1987, 31, 820–829.
- Hiemstra, P.S.; Gorter, A.; Stuurman, M.E.; Van Es, L.A.; Daha, M.R. Activation of the alternative pathway of complement by human serum IgA. Eur. J. Immunol. 1987, 17, 321–326.
- Russell, M.W.; Mansa, B. Complement-fixing properties of human IgA antibodies. Alternative pathway complement activa-tion by plastic-bound, but not specific antigen-bound, IgA. Scand. J. Immunol. 1989, 30, 175–183.
- Chiu, Y.-L.; Lin, W.-C.; Shu, K.-H.; Fang, Y.-W.; Chang, F.-C.; Chou, Y.-H.; Wu, C.-F.; Chiang, W.-C.; Lin, S.-L.; Chen, Y.-M.; et al. Alternative Complement Pathway Is Activated and Associated with Galactose-Deficient IgA1 Antibody in IgA Nephropathy Patients. Front. Immunol. 2021, 12.
- Medjeral-Thomas, N.R.; Moffitt, H.; Lomax-Browne, H.J.; Constantinou, N.; Cairns, T.; Cook, H.T.; Pickering, M.C. Glomerular Complement Factor H–Related Protein 5 (FHR5) Is Highly Prevalent in C3 Glomerulopathy and Associated With Renal Impairment. Kidney Int. Rep. 2019, 4, 1387–1400.
- Zhang, J.-J.; Jiang, L.; Liu, G.; Wang, S.-X.; Zou, W.-Z.; Zhang, H.; Zhao, M.-H. Levels of Urinary Complement Factor H in Patients with IgA Nephropathy are Closely Associated with Disease Activity. Scand. J. Immunol. 2009, 69, 457–464.
- Miyazaki, R.; Kuroda, M.; Akiyama, T.; Otani, I.; Tofuku, Y.; Takeda, R. Glomerular deposition and serum levels of complement control proteins in patients with IgA nephropathy. Clin. Nephrol. 1984, 21, 335–340.
- Tomino, Y.; Endoh, M.; Nomoto, Y.; Sakai, H. Double immunofluorescence studies of immunoglobulins, complement components and their control proteins in patients with IgA nephropathy. Pathol. Int. 1982, 32, 251–256.
- Tomino, Y.; Sakai, H.; Nomoto, Y.; Endoh, M.; Arimori, S.; Fujita, T. Deposition of C4-binding protein and β 1H globulin in kidneys of patients with IgA nephropathy. Tokai J. Exp. Clin. Med. 1981, 6, 217–222.
- Onda, K.; Ohsawa, I.; Ohi, H.; Tamano, M.; Mano, S.; Wakabayashi, M.; Toki, A.; Horikoshi, S.; Fujita, T.; Tomino, Y. Excretion of complement proteins and its activation marker C5b-9 in IgA nephropathy in relation to renal function. BMC Nephrol. 2011, 12, 64.
- Wen, L.; Zhao, Z.; Wang, Z.; Xiao, J.; Birn, H.; Gregersen, J.W. High levels of urinary complement proteins are associated with chronic renal damage and proximal tubule dysfunction in immunoglobulin A nephropathy. Nephrology 2018, 24, 703–710.
- Liu, M.; Chen, Y.; Zhou, J.; Liu, Y.; Wang, F.; Shi, S.; Zhao, Y.; Wang, S.; Liu, L.; Lv, J.; et al. Implication of Urinary Complement Factor H in the Progression of Immunoglobulin A Nephropathy. PLoS ONE 2015, 10, e0126812.
- Poppelaars, F.; de Jorge, E.G.; Jongerius, I.; Baeumner, A.J.; Steiner, M.-S.; Józsi, M.; Toonen, E.J.M.; Pauly, D. The SciFiMed consortium A Family Affair: Addressing the Challenges of Factor H and the Related Proteins. Front. Immunol. 2021, 12, 12.
- Paunas, T.I.F.; Finne, K.; Leh, S.; Marti, H.-P.; Mollnes, T.E.; Berven, F.; Vikse, B.E. Glomerular abundance of complement proteins characterized by proteomic analysis of laser-captured microdissected glomeruli associates with progressive disease in IgA nephropathy. Clin. Proteom. 2017, 14, 30.
- Murphy, B.; Georgiou, T.; Machet, D.; Hill, P.; McRae, J. Factor H-related protein-5: A novel component of human glomerular immune deposits. Am. J. Kidney Dis. 2002, 39, 24–27.
- Guo, W.-Y.; Sun, L.-J.; Dong, H.-R.; Wang, G.-Q.; Xu, X.-Y.; Zhao, Z.-R.; Cheng, H. Glomerular Complement Factor H–Related Protein 5 is Associated with Histologic Injury in Immunoglobulin A Nephropathy. Kidney Int. Rep. 2021, 6, 404–413.
- Tortajada, A.; Gutiérrez, E.; De Jorge, E.G.; Anter, J.; Segarra, A.; Espinosa, M.; Blasco, M.; Roman, E.; Marco, H.; Quintana, L.F.; et al. Elevated factor H–related protein 1 and factor H pathogenic variants decrease complement regulation in IgA nephropathy. Kidney Int. 2017, 92, 953–963.
- Medjeral-Thomas, N.R.; Lomax-Browne, H.J.; Beckwith, H.; Willicombe, M.; McLean, A.G.; Brookes, P.; Pusey, C.D.; Falchi, M.; Cook, H.T.; Pickering, M.C. Circulating complement factor H–related proteins 1 and 5 correlate with disease activity in IgA nephropathy. Kidney Int. 2017, 92, 942–952.
- Gharavi, A.G.; Kiryluk, K.; Choi, M.; Li, Y.; Hou, P.; Xie, J.; Sanna-Cherchi, S.; Men, C.J.; Julian, B.A.; Wyatt, R.; et al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat. Genet. 2011, 43, 321–327.
- Kiryluk, K.; Li, Y.; Scolari, F.; Sanna-Cherchi, S.; Choi, M.; Verbitsky, M.; Fasel, D.; Lata, S.; Prakash, S.; Shapiro, S.; et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat. Genet. 2014, 46, 1187–1196.
- Kiryluk, K.; Li, Y.; Sanna-Cherchi, S.; Rohanizadegan, M.; Suzuki, H.; Eitner, F.; Snyder, H.J.; Choi, M.; Hou, P.; Scolari, F.; et al. Geographic Differences in Genetic Susceptibility to IgA Nephropathy: GWAS Replication Study and Geospatial Risk Analysis. PLoS Genet. 2012, 8, e1002765.
- Xie, J.; Kiryluk, K.; Li, Y.; Mladkova, N.; Zhu, L.; Hou, P.; Ren, H.; Wang, W.; Zhang, H.; Chen, N.; et al. Fine Mapping Implicates a Deletion of CFHR1 and CFHR3 in Protection from IgA Nephropathy in Han Chinese. J. Am. Soc. Nephrol. 2016, 27, 3187–3194.
- Zhai, Y.-L.; Meng, S.-J.; Zhu, L.; Shi, S.-F.; Wang, S.-X.; Liu, L.-J.; Lv, J.-C.; Yu, F.; Zhao, M.-H.; Zhang, H. Rare Variants in the Complement Factor H–Related Protein 5 Gene Contribute to Genetic Susceptibility to IgA Nephropathy. J. Am. Soc. Nephrol. 2016, 27, 2894–2905.
- Zhu, L.; Guo, W.-Y.; Shi, S.-F.; Liu, L.-J.; Lv, J.-C.; Medjeral-Thomas, N.R.; Lomax-Browne, H.J.; Pickering, M.C.; Zhang, H. Circulating complement factor H–related protein 5 levels contribute to development and progression of IgA nephropathy. Kidney Int. 2018, 94, 150–158.
- Endo, M.; Ohi, H.; Ohsawa, I.; Fujita, T.; Matsushita, M. Glomerular deposition of mannose-binding lectin (MBL) indicates a novel mechanism of complement activation in IgA nephropathy. Nephrol. Dial. Transplant. 1998, 13, 1984–1990.
- Hisano, S.; Matsushita, M.; Fujita, T.; Endo, Y.; Takebayashi, S. Mesangial IgA2 deposits and lectin pathway-mediated complement activation in IgA glomerulonephritis. Am. J. Kidney Dis. 2001, 38, 1082–1088.
- Faria, B.; Henriques, C.; Matos, A.; Daha, M.R.; Pestana, M.; Seelen, M. Combined C4d and CD3 immunostaining predicts immunoglobulin (Ig)A nephropathy progression. Clin. Exp. Immunol. 2015, 179, 354–361.
- Liu, L.-L.; Jiang, Y.; Wang, L.-N.; Liu, N. Urinary mannose-binding lectin is a biomarker for predicting the progression of immunoglobulin (Ig)A nephropathy. Clin. Exp. Immunol. 2012, 169, 148–155.
- Segarra-Medrano, A.; Carnicer-Caceres, C.; Valtierra-Carmeno, N.; Agraz-Pamplona, I.; Terrades, N.R.; Escalante, E.J.; Ostos-Roldan, E. Estudio de las variables asociadas a la activación local del complemento en la nefropatía IgA idiopática. Nefrologia 2017, 37, 320–329.
- Espinosa, M.; Ortega, R.; Gómez-Carrasco, J.M.; López-Rubio, F.; López-Andreu, M.; López-Oliva, M.O.; Aljama, P. Mesangial C4d deposition: A new prognostic factor in IgA nephropathy. Nephrol. Dial. Transplant. 2008, 24, 886–891.
- Haas, M.; Loupy, A.; Lefaucheur, C.; Roufosse, C.; Glotz, D.; Seron, D.; Nankivell, B.J.; Halloran, P.F.; Colvin, R.B.; Akalin, E.; et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell–mediated rejection, anti-body-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am. J. Transplant. 2018, 18, 293–307.
- Baek, H.S.; Han, M.H.; Kim, Y.J.; Cho, M.H. Clinical Relevance of C4d Deposition in Pediatric Immunoglobulin A Nephropathy. Fetal Pediatr. Pathol. 2018, 37, 326–336.
- Jiang, Y.; Zan, J.; Shi, S.; Hou, W.; Zhao, W.; Zhong, X.; Zhou, X.; Lv, J.; Zhang, H. Glomerular C4d Deposition and Kidney Disease Progression in IgA Nephropathy: A Systematic Review and Meta-analysis. Kidney Med. 2021.
- Ohsawa, I.; Kusaba, G.; Ishii, M.; Sato, N.; Inoshita, H.; Onda, K.; Hashimoto, A.; Nagamachi, S.; Suzuki, H.; Shimamoto, M.; et al. Extraglomerular C3 deposition and metabolic impacts in patients with IgA nephropathy. Nephrol. Dial. Transplant. 2012, 28, 1856–1864.
- Medjeral-Thomas, N.R.; Troldborg, A.; Constantinou, N.; Lomax-Browne, H.J.; Hansen, A.G.; Willicombe, M.; Pusey, C.D.; Cook, H.T.; Thiel, S.; Pickering, M.C. Progressive IgA Nephropathy Is Associated With Low Circulating Mannan-Binding Lectin–Associated Serine Protease-3 (MASP-3) and Increased Glomerular Factor H–Related Protein-5 (FHR5) Deposition. Kidney Int. Rep. 2018, 3, 426–438.
- Xu, L.; Yang, H.-C.; Hao, C.-M.; Lin, S.-T.; Gu, Y.; Ma, J. Podocyte number predicts progression of proteinuria in IgA nephropathy. Mod. Pathol. 2010, 23, 1241–1250.
- Moll, S.; Miot, S.; Sadallah, S.; Gudat, F.; Mihatsch, M.J.; Schifferli, J.A. No complement receptor 1 stumps on podocytes in human glomerulopathies. Kidney Int. 2001, 59, 160–168.
- Koopman, J.J.E.; van Essen, M.F.; Rennke, H.G.; de Vries, A.P.J.; van Kooten, C. Deposition of the Membrane Attack Complex in Healthy and Diseased Human Kidneys. Front. Immunol. 2021, 11, 3802.
- Ootaka, T.; Suzuki, M.; Sudo, K.; Sato, H.; Seino, J.; Saito, T.; Yoshinaga, K. Histologic Localization of Terminal Complement Complexes in Renal Diseases: An Immunohistochemical Study. Am. J. Clin. Pathol. 1989, 91, 144–151.
- Bariety, J.; Hinglais, N.; Bhakdi, S.; Mandet, C.; Rouchon, M.; Kazatchkine, M.D. Immunohistochemical study of complement S protein (Vitronectin) in normal and diseased human kidneys: Relationship to neoantigens of the C5b-9 terminal complex. Clin. Exp. Immunol. 1989, 75, 76–81.
- Hinglais, N.; Kazatchkine, M.D.; Bhakdi, S.; Appay, M.; Mandet, C.; Grossetete, J.; Bariéty, J. Immunohistochemical study of the C5b-9 complex of complement in human kidneys. Kidney Int. 1986, 30, 399–410.
- Alexopoulos, E.; Papaghianni, A.; Papadimitriou, M. The pathogenetic significance of C5b-9 in IgA nephropathy. Nephrol. Dial. Transplant. 1995, 10, 1166–1172.
- Stangou, M.; Alexopoulos, E.; Pantzaki, A.; Leonstini, M.; Memmos, D. C5b-9 glomerular deposition and tubular α3β1-integrin expression are implicated in the development of chronic lesions and predict renal function outcome in immunoglobulin A nephropathy. Scand. J. Urol. Nephrol. 2008, 42, 373–380.
- Pratt, J.R.; Abe, K.; Miyazaki, M.; Zhou, W.; Sacks, S.H. In Situ Localization of C3 Synthesis in Experimental Acute Renal Allograft Rejection. Am. J. Pathol. 2000, 157, 825–831.
- Abe, K.; Miyazaki, M.; Koji, T.; Furusu, A.; Shioshita, K.; Tsukasaki, S.; Ozono, Y.; Harada, T.; Sakai, H.; Kohno, S. Intraglomerular synthesis of complement C3 and its activation products in IgA nephropathy. Nephron 2001, 87, 231–239.
- Eguchi, K.; Tomino, Y.; Yagame, M.; Miyazaki, M.; Takiura, F.; Miura, M.; Suga, T.; Endoh, M.; Nomoto, Y.; Sakai, H. Double immunofluorescence studies of IgA and poly C9 (MAC) in glomeruli from patients with IgA nephropathy. Tokai J. Exp. Clin. Med. 1987, 12, 331–335.
- Mosolits, S.; Magyarlaki, T.; Nagy, J. Membrane Attack Complex and Membrane Cofactor Protein Are Related to Tubulointerstitial Inflammation in Various Human Glomerulopathies. Nephron 1997, 75, 179–187.
- Dumont, C.; Mérouani, A.; Ducruet, T.; Benoit, G.; Clermont, M.-J.; Lapeyraque, A.L.; Phan, V.; Patey, N. Clinical relevance of membrane attack complex deposition in children with IgA nephropathy and Henoch-Schönlein purpura. Pediatr. Nephrol. 2020, 35, 843–850.
- Takahashi, T.; Inaba, S.; Okada, T. Vitronectin in children with renal disease—1. Immunofluorescence study of vitronectin and C5b-9 in childhood IgA nephropathy. Nihon Jinzo Gakkai Shi 1995, 37, 213–223.
- Liu, L.; Zhang, Y.; Duan, X.; Peng, Q.; Liu, Q.; Zhou, Y.; Quan, S.; Xing, G. C3a, C5a Renal Expression and Their Receptors are Correlated to Severity of IgA Nephropathy. J. Clin. Immunol. 2014, 34, 224–232.
- Tanaka, C.; Suhara, Y.; Kikkawa, Y. Circulating immune complexes and complement breakdown products in childhood IgA nephropathy. Nihon Jinzo Gakkai Shi 1991, 33, 709–717.
- Wyatt, R.J.; Julian, B.A. Activation of Complement in IgA Nephropathy. Am. J. Kidney Dis. 1988, 12, 437–442.
- Zwirner, J.; Burg, M.; Schulze, M.; Brunkhorst, R.; Götze, O.; Koch, K.-M.; Floege, J. Activated complement C3: A potentially novel predictor of progressive IgA nephropathy. Kidney Int. 1997, 51, 1257–1264.
- Wyatt, R.; Kanayama, Y.; Julian, B.A.; Negoro, N.; Sugimoto, S.; Hudson, E.C.; Curd, J.G. Complement activation in IgA nephropathy. Kidney Int. 1987, 31, 1019–1023.
- Knoppova, B.; Reily, C.; Maillard, N.; Rizk, D.V.; Moldoveanu, Z.; Mestecky, J.; Raska, M.; Renfrow, M.B.; Julian, B.A.; Novak, J. The Origin and Activities of IgA1-Containing Immune Complexes in IgA Nephropathy. Front. Immunol. 2016, 7, 117.
- Yang, X.; Wei, R.-B.; Wang, Y.; Su, T.-Y.; Li, Q.-P.; Yang, T.; Huang, M.-J.; Li, K.-Y.; Chen, X.-M. Decreased Serum C3 Levels in Immunoglobulin A (IgA) Nephropathy with Chronic Kidney Disease: A Propensity Score Matching Study. Med. Sci. Monit. 2017, 23, 673–681.
- Kawasaki, Y.; Maeda, R.; Ohara, S.; Suyama, K.; Hosoya, M. Serum IgA/C3 and glomerular C3 staining predict severity of IgA nephropathy. Pediatr. Int. 2017, 60, 162–167.
- Mizerska-Wasiak, M.; Małdyk, J.; Rybi-Szuminska, A.; Wasilewska, A.; Miklaszewska, M.; Pietrzyk, J.; Firszt-Adamczyk, A.; Stankiewicz, R.; Bieniaś, B.; Zajączkowska, M.; et al. Relationship between serum IgA/C3 ratio and severity of histological lesions using the Oxford classification in children with IgA nephropathy. Pediatr. Nephrol. 2015, 30, 1113–1120.
- Chen, P.; Yu, G.; Zhang, X.; Xie, X.; Wang, J.; Shi, S.; Liu, L.; Lv, J.; Zhang, H. Plasma Galactose-Deficient IgA1 and C3 and CKD Progression in IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2019, 14, 1458–1465.
- Onda, K.; Ohi, H.; Tamano, M.; Ohsawa, I.; Wakabayashi, M.; Horikoshi, S.; Fujita, T.; Tomino, Y. Hypercomplementemia in adult patients with IgA nephropathy. J. Clin. Lab. Anal. 2007, 21, 77–84.
- Thurman, J.M.; Laskowski, J. Complement factor H–related proteins in IgA nephropathy—sometimes a gentle nudge does the trick. Kidney Int. 2017, 92, 790–793.
- Guo, W.-Y.; Zhu, L.; Meng, S.-J.; Shi, S.-F.; Liu, L.-J.; Lv, J.-C.; Zhang, H. Mannose-Binding Lectin Levels Could Predict Prognosis in IgA Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 3175–3181.
- Degn, S.E.; Thiel, S.; Nielsen, O.; Hansen, A.G.; Steffensen, R.; Jensenius, J.C. MAp19, the alternative splice product of the MASP2 gene. J. Immunol. Methods 2011, 373, 89–101.
- Martin, M.; Trattner, R.; Nilsson, S.C.; Björk, A.; Zickert, A.; Blom, A.M.; Gunnarsson, I. Plasma C4d Correlates with C4d Deposition in Kidneys and With Treatment Response in Lupus Nephritis Patients. Front. Immunol. 2020, 11, 582737.
- Dobó, J.; Szakács, D.; Oroszlán, G.; Kortvely, E.; Kiss, B.; Boros, E.; Szász, R.; Závodszky, P.; Gál, P.; Pál, G. MASP-3 is the exclusive pro-factor D activator in resting blood: The lectin and the alternative complement pathways are fundamentally linked. Sci. Rep. 2016, 6, 31877.
- Kiryluk, K.; Novak, J. The genetics and immunobiology of IgA nephropathy. J. Clin. Investig. 2014, 124, 2325–2332.
- Yu, X.-Q.; Li, M.; Zhang, H.; Low, H.-Q.; Wei, X.; Wang, J.-Q.; Sun, L.-D.; Sim, K.S.; Li, Y.; Foo, J.N.; et al. A genome-wide association study in Han Chinese identifies multiple susceptibility loci for IgA nephropathy. Nat. Genet. 2012, 44, 178–182.
- Holmes, L.V.; Strain, L.; Staniforth, S.J.; Moore, I.; Marchbank, K.; Kavanagh, D.; Goodship, J.A.; Cordell, H.J.; Goodship, T.H.J. Determining the Population Frequency of the CFHR3/CFHR1 Deletion at 1q32. PLoS ONE 2013, 8, e60352.
- Zhao, J.; Wu, H.; Khosravi, M.; Cui, H.; Qian, X.; Kelly, J.; Kaufman, K.M.; Langefeld, C.D.; Williams, A.H.; Comeau, M.E.; et al. Association of Genetic Variants in Complement Factor H and Factor H-Related Genes with Systemic Lupus Erythematosus Susceptibility. PLoS Genet. 2011, 7, e1002079.
- Zipfel, P.F.; Edey, M.; Heinen, S.; Józsi, M.; Richter, H.; Misselwitz, J.; Hoppe, B.; Routledge, D.; Strain, L.; Hughes, A.E.; et al. Deletion of Complement Factor H–Related Genes CFHR1 and CFHR3 Is Associated with Atypical Hemolytic Uremic Syndrome. PLoS Genet. 2007, 3, e41.
- Hughes, A.E.; Orr, N.; Esfandiary, H.; Diaz-Torres, M.; Goodship, T.; Chakravarthy, U. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat. Genet. 2006, 38, 1173–1177.
- Zhu, L.; Zhai, Y.-L.; Wang, F.-M.; Hou, P.; Lv, J.-C.; Xu, D.-M.; Shi, S.-F.; Liu, L.-J.; Yu, F.; Zhao, M.-H.; et al. Variants in Complement Factor H and Complement Factor H-Related Protein Genes, CFHR3 and CFHR1, Affect Complement Activation in IgA Nephropathy. J. Am. Soc. Nephrol. 2014, 26, 1195–1204.
- Jullien, P.; Laurent, B.; Claisse, G.; Masson, I.; Dinic, M.; Thibaudin, D.; Berthoux, F.; Alamartine, E.; Mariat, C.; Maillard, N. Deletion Variants of CFHR1 and CFHR3 Associate with Mesangial Immune Deposits but Not with Progression of IgA Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 661–669.
- Pesce, F.; Stea, E.D.; Divella, C.; Accetturo, M.; Laghetti, P.; Gallo, P.; Rossini, M.; Cianciotta, F.; Crispino, L.; Granata, A.; et al. DelCFHR3-1 influences graft survival in transplant patients with IgA nephropathy via complement-mediated cellular senescence. Arab. Archaeol. Epigr. 2021, 21, 838–845.
- Garred, P. Mannose-binding lectin deficiency—Revisited. Mol. Immunol. 2003, 40, 73–84.
- Garred, P.; Larsen, F.; Seyfarth, J.; Fujita, R.; Madsen, H.O. Mannose-binding lectin and its genetic variants. Genes Immun. 2006, 7, 85–94.
- Gong, Z.L.R. Mannose-binding Lectin Gene Polymorphism Associated with the Patterns of Glomerular Immune Deposition in IgA Nephropathy. Scand. J. Urol. Nephrol. 2001, 35, 228–232.
- Shi, B.; Wang, L.; Mou, S.; Zhang, M.; Wang, Q.; Qi, C.; Cao, L.; Che, X.; Fang, W.; Gu, L.; et al. Identification of mannose-binding lectin as a mechanism in progressive immunoglobulin A nephropathy. Int. J. Clin. Exp. Pathol. 2015, 8, 1889–1899.
- Ouyang, Y.; Zhu, L.; Shi, M.; Yu, S.; Jin, Y.; Wang, Z.; Ma, J.; Yang, M.; Zhang, X.; Pan, X.; et al. A Rare Genetic Defect of MBL2 Increased the Risk for Progression of IgA Nephropathy. Front. Immunol. 2019, 10.
