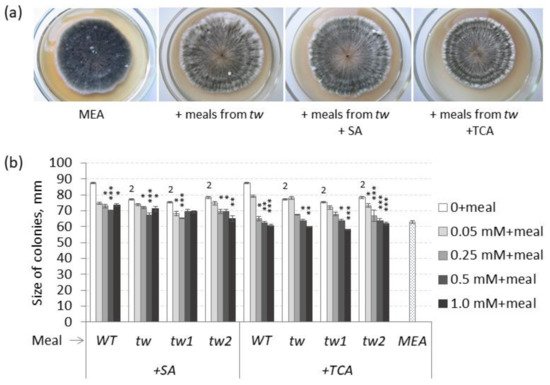
2.1. The Action of 2,4-D, SA and TCA on MGG and Root Growth of Allelic Tweaky Spike Mutants
Naturally, grain sterilization before germination is an effective treatment against MGG. In most cases studied, the MGG level was significantly lower in surface-sterilized grains than in unsterilized grains under the same experimental conditions, and independent of the plant genotype and the studied 2,4-D concentrations (
Figure 1a and
Figure 2a,
Tables S1a and S2a).
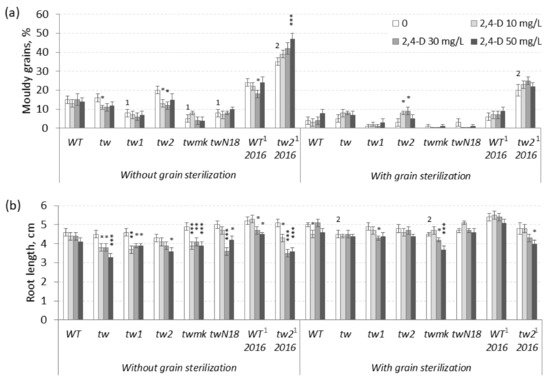
Figure 1. Effects of 2,4-D over a 10–50 mg L−1 range on barley tweaky-type mutants. (a) The frequency of mouldy grains (%) and (b) the root lengths (cm) of unsterilized and sterilized germinating grains measured after 5 days. For mouldy grains, n = 10 Petri dishes (100 grains); for root length n = 30; for tw2, n = 15 Petri dishes (150 grains) and n = 60. WT2016 and tw22016, grain reproduction in 2016; other material, grain reproduction in 2013; the MGG assay was performed in 2017. The asterisks represent significant differences (* p < 0.05; ** p < 0.01; *** p < 0.001) between the control and the 2,4-D treatment. The numbers denote significant differences (1 p < 0.05; 2 p < 0.01) between the controls of the tw-type mutant and the WT.
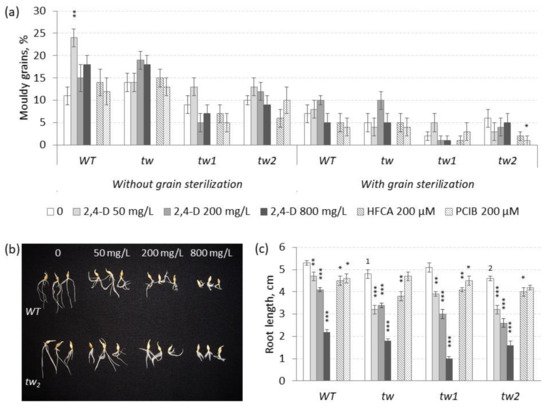
Figure 2. Effects of 2,4-D over a range of 50–800 mg L−1 and the auxin inhibitors HFCA and PCIB on (a) the frequency of mouldy grains (%) and (b,c) the root length (cm) of unsterilized germinating grains of tw mutants and the WT, as measured after 5 days. For mouldy grains, n = 10 Petri dishes (100 grains); for root length, n = 30. Grain was produced in 2013, and the MGG assay was performed in 2017. The asterisks represent significant differences (* p < 0.05; ** p < 0.01; *** p < 0.001) between the control and the 2,4-D treatment. The numbers denote significant differences (1 p < 0.05; 2 p < 0.01) between the controls of the tw-type mutant and the WT.
In the range of 10–50 mg L
−1, an appreciable inhibitory effect of 2,4-D on the root growth of allelic
tw-type mutants depended on (1) the plant genotype and (2) the sterilization status of grains (
Figure 1b and
Table S1b). In most cases, 2,4-D significantly inhibited root growth only in unsterilized germinating grains of all tested genotypes, including the nonallelic
twN18 and
twmk mutants. The
twmk mutant was the most sensitive to 2,4-D among all tested
tweaky-type mutants. In contrast, 2,4-D-induced root growth inhibition in sterilized germinating grains mostly in a nonsignificant manner (
Figure 1b and
Table S1b).
The tested lower 2,4-D concentrations (10–50 mg L
−1) revealed an interesting dependence of 2,4-D-induced root growth inhibition on grain sterilization status; consequently, the effect of elevated 2,4-D concentrations was investigated in further experiments. In the range of 50–800 mg L
−1 2,4-D, a significant effect of 2,4-D on MGG was observed only in unsterilized grains of
WT (
p = 0.0026), in which 2,4-D increased the level of MGG (
Figure 2a and
Table S2a), while in the range of 10–50 mg L
−1 2,4-D, a significant increase in MGG was observed only in sterilized grains of the
tw2 genotype (
p = 0.024;
Figure 1a and
Table S1a).
In contrast to the effect of the lower concentrations, the higher concentrations (50–800 mg L
−1) of 2,4-D induced a significant decrease in root length independent of the plant genotype and the grain sterilization conditions, and the inhibitory effect of 2,4-D on root growth was dose-dependent (
Figure 2b,c and
Table S2b). The root growth of the
tw and
tw2 allelic mutants, but not the
tw1 allelic mutant, was weaker than that of
WT germinating grains. An unsterilized grain background better revealed the inhibitory effect of 2,4-D on the germination rate, which was uniform independent of the plant genotype. In turn, grain sterilization revealed better differences among allelic mutants in the response to 2,4-D according to the germination rate (
Table S2c). In general, 2,4-D in the range of 50–800 mg L
−1 inhibited root length independent of the plant genotype (
Figure 2c), while in the range of 10–50 mg L
−1 2,4-D, differences between
WT and
tw-type mutants and among allelic
tw mutants themselves were observed (
Figure 1).
Despite the proposed opposite effects to the action of 2,4-D, the auxin inhibitors HFCA and PCIB did not show a significant effect on MGG, except PCIB in sterilized grains of the
tw2 mutant, in which the MGG level decreased (
Figure 2a and
Table S2a). However, both auxin inhibitors suppressed root growth similarly to 2,4-D (
Figure 2c and
Table S2b).
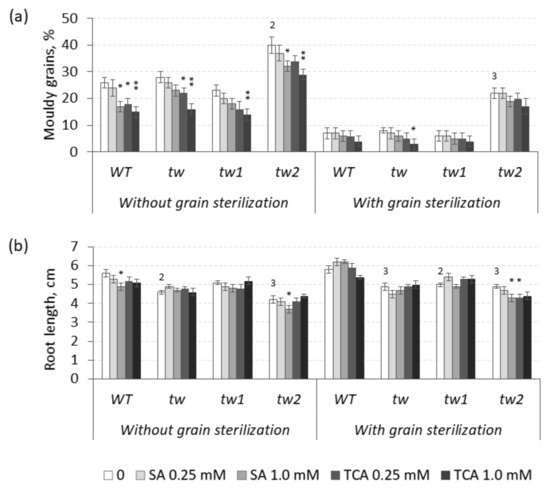
Theoretically, the effects of TCA and SA are supposed to be opposite to those of 2,4-D, and TCA decreased the frequency of MGG in unsterilized germinating grains of the
WT and all allelic
tw-type mutants (
Figure 3 and
Table S3).
Figure 3. Effects of salicylic (SA) and trans-cinnamic (TCA) acids on (a) the frequency of mouldy grains (%) and (b) the root lengths (cm) of unsterilized and sterilized germinating grains of barley tw mutants and the WT, as measured after 5 days. For mouldy grains, n = 10 (100 grains); for root length, n = 30. Grain was produced in 2016, and the MGG test was performed in 2020. The asterisks represent significant differences (* p < 0.05; ** p < 0.01) between the control and the SA or TCA treatment. The numbers denote significant differences (2 p < 0.01; 3 p < 0.001) between the controls of the tw-type mutant and the WT.
However, in sterilized germinating grains, TCA decreased MGG at a significant level only in the allelic
tw mutant, while SA decreased MGG at a significant level only in unsterilized grains of the
tw2 mutant (
Figure 3 and
Table S3a). Similar to 2,4-D, SA and TCA also inhibited root growth, but only in the allelic mutant
tw2 (
Figure 3b and
Table S3b). Neither compound showed any effect on the grain germination rate (
Table S3c).
2.2. Fungi Spectrum in the Internal Grain Tissues of Barley tw-Type Mutants and Revertants
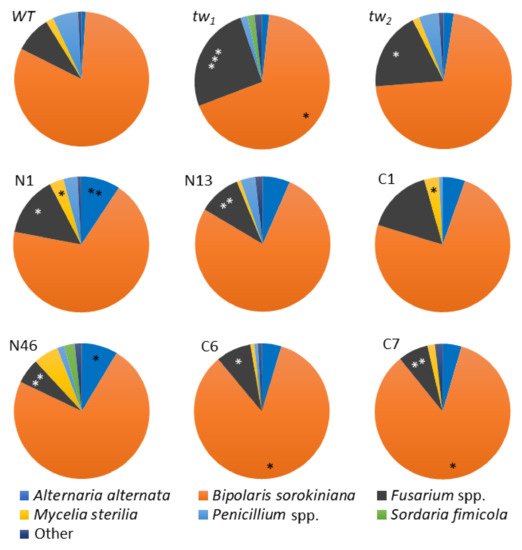
To reveal the possible differences in the fungal diversity in surface-sterilized mouldy germinating grains of tested barley genotypes, the spectrum of fungi species was investigated. Among the fungi that frequently reside in the internal tissues of barley grains,
B. sorokiniana prevailed in all the tested plant genotypes (
Figure 4 and
Table S4). In addition to the
WT,
tw1 and
tw2 mutants, the fungi spectra were also studied in several revertants that arose during the phase of stabilization of
tw1 and
tw2 mutants. The revertant studies allowed for a broader understanding of the differences between
tw-type mutants and the
WT.
Figure 4. Spectra of fungi (%) in the internal grain tissues of barley tw mutants and the WT. The middle row—revertants from tw1; the lower row—revertants from tw2. N—revertants with normal spike and floral structure, C—revertants with normal floral structure but compactoid spikes. Grain was produced in 2013, and the analysis was performed in 2014. The asterisks represent significant differences (* p < 0.05; ** p < 0.01; *** p < 0.001) between the tw-type mutant and the WT (in the upper row) or between the revertant and the respective initial tw mutant (N1, N13 and C1 derived from tw1, N46, C6 and C7—from tw2).
In the grains of the
tw1 and
tw2 mutants, the
B. sorokiniana frequency was lower than that in the
WT strain, but the difference was only statistically significant in
tw1. Interestingly, the level of
B. sorokiniana was only significantly lower in grains of revertant N1 compared to
WT (
p < 0.05) and remained the same as in grains of its parental mutant
tw1. Such a tendency was not observed. A similar tendency was also observed in other revertants, except for the compactoid (C)-type revertants from
tw2, but only in an insignificant manner. However, a decrease in the
Bipolaris proportion occurred at the expense of the increasing
Fusarium portion in the fungi spectra, and the observed effect was statistically significant (
Figure 4). Comparable results were also obtained after analysing the fungi spectra in the internal grain tissues in our previous studies [
22]. This finding provided a pretext for studying the effects of grain meals made from the different
tw-type allelic mutants and the
WT on
B. sorokiniana growth.