Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Heart failure (HF) is a rapidly growing global public health problem. Since HF results in high mortality and re-hospitalization, new effective treatments are desired. Although it remains controversial, omega 3 polyunsaturated fatty acids (n-3 PUFAs), such as the eicosapentaenoic acid and docosahexaenoic acid, have been widely recognized to have benefits for HF.
- omega 3 polyunsaturated fatty acid
- Heart failure
- eicosapentaenoic acid
- docosahexaenoic acid
- cardiovascular disease
1. Introduction
Heart failure (HF) is a rapidly growing global public health problem both in developed and developing countries, with an estimated prevalence of over 37.7 million patients worldwide [1]. Despite recent developments of HF treatments, including pharmacologic and device therapy, HF results in high mortality and re-hospitalization. Therefore, innovations regarding HF treatments are desired.
2. n-3 PUFA on Heart Failure
HF is a condition in which the heart is unable to pump enough blood to meet demand. Since HF is a multifactorial syndrome, its mortalities and progressions differ from their underling etiologies, such as IHD, valvular heart disease, hypertension, arrhythmia, cardiac myopathies, congenital heart disease, endocrine and metabolic diseases, infection, and certain drugs. Several prior prospective observational studies and randomized control trials proved that consumption of fish or fish oil containing n-3 PUFA decreased IHD mortality (e.g., myocardial infarction (MI) and sudden cardiac death) in patients with or without pre-diagnosed CVD [2,19,20]. In addition, recent studies have shown that fish and/or n-3 PUFA intake also prevented the new onset of HF and rehospitalization [9,10,21,22,23,24]. However, conflicting results were reported from other groups [25,26,27], meaning that the n-3 PUFA-mediated effects on HF are once again viewed as uncertain. In this section, we summarize existing clinical evidence regarding primary and secondary prevention of HF, and discuss the considerable points which may cause such heterogenous results.
3. n-3 PUFA-Mediated Cardiac Protection from Basic and Translational Research
Despite controversy regarding the benefits of n-3 PUFA for HF prevention in clinical trials, a significant amount of supportive evidence from basic and clinical translational research has been reported. Although such follow-up research tends to contain an inherent bias, by which positive data are likely to be reported, each study has well-explained pathophysiological mechanisms of preventive effects of PUFA against HF (Figure 2). In this section, we summarize the evidence of n-3 PUFA-mediated cardiac protection and HF prevention both from basic and translational research.
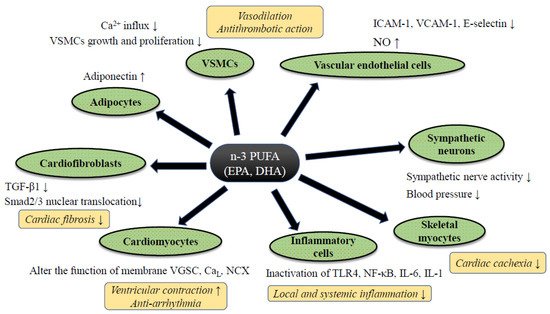
Figure 2. The putative mechanism of n-3 PUFA-mediated cardiac protection against heart failure. Abbreviations: CaL; L type calcium channel, IL-6/-1; ICAM-1; intercellular adhesion molecule-1, Interleukin-6/-1, NCX: sodium calcium exchanger, NF-κB; nuclear factor-kappa B, NO; nitric oxide, TGF-β1; transforming growth factor-beta 1, TLR4; Toll-like receptor 4, VGSC; voltage-gated sodium channel, VCAM-1; vascular cell adhesion molecule-1, VSMCs; vascular smooth muscle cells. Abbreviations: ADMA: Asymmetric Dimethylarginine, DB: Double blind trial, ESLVD: End-systolic left ventricle diameters, Etv/Atv: Early rapid right ventricular filling/late right ventricular filling, FMD: Flow-mediated dilatation, LAEF: Left atrial ejection fraction, LVESVI: Left ventricular end-systolic volume indexed to body surface area, MC: Multi-center trial, MCP-1: Monocyte chemoattractant protein 1, NYHA: New York Heart Association class, OL: Open label trial, PC: Placebo-control trial, PRSP: Prospective trial, RDM: Randomized trial, SC: Single-center trial, ST2: Suppression of tumorigenicity 2, TDI: Tissue Doppler imaging. Downward allows represent the decrease or suppression, and upward allows the increase or enhancement.
3.1. Anti-Inflammatory Effect of n-3 PUFA
It is well known that the inflammatory cytokines (e.g., interleukin-1β and TNF-α), which can reduce both systolic and diastolic cardiac function to advance cardiac remodeling through abnormal calcium handling in cardiomyocytes and enhancement of cardiac fibrosis via an activation of fibroblasts, are elevated both in the blood and local heart tissue of HF patients [58]. In addition, systemic inflammatory cytokines are considered one of the major causes of cachexia during HF [59,60]. Thus, the activation of local and/or systemic inflammatory reaction plays a pivotal role for the pathogenesis and progression of HF.
Classically, eicosanoids originated from n-3 PUFA are known to enhance the production of anti-inflammatory cytokines at the site of inflammation, whereas essential n-6 PUFAs, such as AA, are considered as substrates of pro-inflammatory eicosanoids, which promote vascular permeability, leucocyte infiltration and activation, and pro-inflammatory cytokine release [61]. The conventional interpretation is that the n-3 PUFAs antagonize the production and action of the inflammatory eicosanoid derived from AA metabolites. Indeed, in a pressure-overloaded HF rodent model (induced by aortic contraction surgery), the n-3 PUFA supplementation reduced serum TNF-α as well as pro-inflammatory eidosanoid (i.e., thromboxane B2), and prevented abnormal LV remodeling [62]. In addition, a number of human clinical investigations revealed that n-3 PUFA supplementation reduced the pro-inflammatory cytokines in terms of lower circulating levels of cytokines, e.g., TNF-α, IL-1, and IL-6 [24,32,63,64]. Basic studies also support the idea that n-3 PUFA would downregulate the pro-inflammatory pathways, such as NF-κB [65] and NLRP3 inflammasome [66], or upregulate anti-inflammatory intra-cellular signaling pathways, including peroxisome proliferator-activated receptor (PPAR) α/γ transcriptional activation [67]. Meanwhile, another study suggested the effect of low dose n-3 PUFA on NF-κB pathway activation in a cultured macrophage, potentially enhancing pro-inflammatory cytokines production [68]. The immunomodulatory activity of n-3 PUFAs has not been clearly explained yet and should be investigated further in detail.
Adiponectin, a peptide hormone released from adipose tissue, is known to show a cardio-protective effect through anti-inflammatory reaction. PPARγ-dependent adiponectin secretion is thought to correlate with n-3 PUFA-mediated cardiac protection, and the administration of n-3 PUFA increased the circulation of adiponectin in a dose-dependent manner both in animal [62,69] and human [70] studies.
The free FA specific membrane receptor family, a family of orphan G-protein coupled receptors (GPR), has been detected in the last 10 years. GPR120, also known as a free fatty acid receptor (FFR) 4, was detected as a receptor for n-3 PUFA, regulating downstream of intra-cellular signaling [71]. Eclov et al. revealed that the expression of FFR4 was markedly higher than other subtypes of FFRs in cardiomyocytes as well as fibroblasts isolated from mice [72]. In a rat heart study, a high-fat diet increased the expression level of FFR4 [73]. The involvement of FFR4 in the n-3 PUFA-mediated cardiac protection has been actively investigated in cardiac fibroblasts rather than cardiac myocytes. In a rodent pressure-overloaded HF model, EPA inhibited the transforming growth factor-beta 1 (TGF-β1) pro-fibrotic pathway via FFR signaling in cardiac fibroblasts and suppressed the entire cardiac fibrosis without requirement of EPA localization into the cellular membrane [72]. In addition, the activation of FFR4 by n-3 PUFA further stimulated endothelial nitric oxide synthase (eNOS) and promoted intracellular nitric oxide (NO), which leads to the suppression of TGF-β1 induced smad2/3 nuclear translocation and inhibition of pro-fibrotic gene transcription. It remains to be elucidated whether FFR4 is also expressed in human cardiomyocytes or cardiac fibroblasts.
In order to obtain enhanced benefits of n-3 PUFA-mediated cardiac protection, it would be better to identify the effective n-3 PUFA metabolites rather than to take a high dose of n-3 PUFA. Recently, the essential PUFA derived large class of cell signaling lipid mediators, named specialized pro-resolving mediators (SPMs; resolvins, protectins, lipoxins, and maresins) were discovered and indicated to exert the resolution of inflammatory reactions at nanomolar levels of concentration [74,75]. Halade et al., in a mouse coronary ligation model, reported that leukocytes, which immigrate from the splenic reservoir to the acutely infarcted myocardium, express lipoxygenases and abundant SPMs, predominantly derived from DHA to resolve the local acute inflammatory reaction [76]. Other rodent studies investigating the post-MI rodent heart revealed that the exogenous delivery of resolvins reduced infarction size [77] and excessive inflammation and fibrosis [78], leading to improved cardiac function [78]. Precursor of resolvins (i.e., 18-hydroxyeicosapentaenoic acid) administration in a pressure overload HF model mouse was also reported to ameliorate cardiac inflammation and fibrosis and preserve systolic function [79]. A recent clinical study revealed that plasma resolvin D1 levels were markedly decreased in patients with chronic HF compared with healthy subjects, suggesting a defect of resolvin biosynthesis in HF conditions [80]. To date, medicine purified or produced from SPMs has not been available or reached the stage of clinical application.
3.2. Effects on Myocardial Energy Metabolism and Mitochondrial Function of n-3 PUFA
Alteration of energetic substrate utilization in myocardium and modification of its intra-cellular signaling pathway also play important roles for the pathogenesis and progression of HF [81]. Previous animal studies revealed that the dietary n-3 PUFA intake altered the mitochondrial membrane phospholipid composition in cardiomyocytes, which led to a decrease in myocardial oxygen consumption without loss of ventricular power generation [82,83]. Furthermore, mitochondrial permeability transition pore (mPTP) opening, by which mitochondrial swelling and apoptotic cell death are activated, is suppressed by n3-PUFA (especially by DHA) supplementation [84,85,86]. Thus, it is considered that n-3 PUFA could protect the heart through improvement in the cardiac mitochondrial function as well as the efficiency of the ATP production [82,83,84,85,86].
In HF, lipotoxicity by excessive serum free FAs, which is caused by chronic adrenergic stimulation, is one of the critical pathophysiological mechanisms to exacerbate HF. Excessive free FA exposure to cardiomyocytes causes uncoupled mitochondrial respiration and reactive oxygen species (ROS) production [87], which thereby causes energy depletion and further impairs contraction in the failing heart [88]. Saturated fatty acids (SFA) (e.g., palmitate and stearate) are reported as the main components of serum free FAs [89]. Since unsaturated FAs, especially n-3 PUFA, are reported to act counter to the behavior of SFA, it is widely accepted that n-3 PUFA may mitigate SFA-induced lipotoxicity.
Mitochondria are dynamic organelles, which can continuously alter their morphology to maintain a number of cellular processes, such as cell cycle, immunity, apoptosis, and mitochondrial quality control. Mitochondrial dynamics play key roles for the pathophysiology of HF [90,91]. Specific fusion-related (e.g., mitofusin 1, mitofusin 2, and optic atrophy 1) and fission-related (e.g., dynamin related protein 1 (Drp1), mitochondrial fission 1 protein, and mitochondrial fission factor) proteins are involved in the regulation of mitochondrial dynamics [92]. We, in our previous investigation, reported that EPA activated phosphorylation of AMP-activated protein kinase (AMPK; a key enzyme for cellular energy homeostasis), altered mitochondrial morphology (relatively elongated mitochondria by suppression in Drp1) in myocardium, and thereby protected myocytes from SFA-induced cardiac lipotoxicity [93]. Despite the attractive pathophysiological findings of n-3 PUFA for cardiac protection against lipotoxicity, further in vivo animal and/or human investigations are warranted.
3.3. Anti-Arrhythmic Property by n-3 PUFA
Patients with HF are highly comorbid with arrhythmia, and the incidence of arrhythmia certainly worsens the rate of mortality of HF [94]. n-3 PUFAs are reported to reduce both atrial and ventricular arrhythmia, which may cause an improvement in the mortality of patients with HF. In the GISSI-HF trial, the reason for CVD risk reduction was mainly presumed to be due to anti-arrhythmic effects [10]. A number of basic experimental studies have revealed direct and/or indirect alteration in the electrophysiological behavior of plasma membrane ion channels of cardiomyocytes, such as the sodium, potassium and calcium channels, as well as the sodium-calcium exchanger [95,96]. In isolated mammalian cardiomyocytes (e.g., neonatal and adult rat ventricular myocytes), n-3 PUFA exhibited the inhibition of sodium current in a dose-dependent manner [97,98]. In addition, n-3 PUFA suppressed an intracellular Ca2+ wave, which was propagated from sarcoplasmic reticulum (SR) Ca2+ release by isoproterenol stimulation [99], suggesting a contribution of n-3 PUFA to suppression of arrhythmogenicity (i.e., myocardial triggered activity and abnormal automaticity) during HF.
Although the detailed mechanism remains unknown, n-3 PUFA may be able to improve the autonomic nervous system. An impaired autonomic tone is one risk-factor of a fatal arrhythmic event and sudden cardiac death in patients with dilated cardiomyopathy [100] and ischemic cardiomyopathy [101]. Some clinical studies revealed that n-3 PUFA supplementation improved heart rate variability (an index of autonomic tone) in patients with ischemic and non-ischemic cardiomyopathy [39,40,102,103]. Thus, n-3 PUFA may improve the mortality of HF patients through the autonomic nervous system and thereby suppression of anti-arrhythmogenicity.
3.4. Anti-Hypertensive Effect, Improvement of Vascular Endothelial Function, and Modulation of Autonomic Nervous System Activity by n-3 PUFA
It is well known that hypertension is a critical factor in developing HF. Previous investigations revealed the beneficial effects of fish oil on hypertension. Morris et. al. reported that the intake of fish oils reduced blood pressure (BP) by 3.0 mmHg in systole and by 1.5 mmHg in diastole (95% CI: systolic BP (4.5–1.5), diastolic BP (2.2–0.8)) [6]. Geleijnse et. al. also revealed that fish oils (mean intake 3.7 g/day) reduced BP by 2.1 mmHg in systole and 1.6 mmHg in diastole (95% CI: systolic BP (1.0–3.2), p < 0.01, diastolic BP (1.0–2.2), p < 0.01), and this anti-hypertensive effect of fish oil was obvious especially in the elderly and patients with hypertension [104]. The n-3 PUFA has been reported to release NO from vascular endothelial cells both in vivo [105] and in vitro [106]. In addition, DHA is reported to activate NO synthase and concentration of tetrahydrobiopterin in the central nervous system, which may increase local NO availability and exert tonic inhibition of central sympathetic outflow [107]. Thus, n-3 PUFA may suppress the progression of HF, not only through BP lowering, but also the correction of autonomic imbalance.
3.5. Anti-Thrombotic and Anti-Atherosclerotic Effects and Prevention of HF by n-3 PUFA
The anti-thrombotic and anti-atherosclerotic effects by n-3 PUFA can contribute to HF prevention through the risk reduction of ischemic heart disease [3,16]. The n-3 PUFA is reported to suppress the synthesis of platelet-derived thromboxane A2 (TXA2), which causes platelet aggregation and vasoconstriction [108], and to increase the plasminogen activator inhibitor-1 with reduction of fibrinogen [109]. Atherosclerotic plaque stabilization by n-3 PUFA has been reported. Matsumoto et al. reported that EPA significantly suppressed the development of atherosclerotic lesions in atherosclerosis-prone mice with reduced production of matrix metalloproteinases released by macrophages in a PPARα-dependent fashion [110]. RCTs of patients awaiting carotid endarterectomy also revealed that the supplementation of n-3 PUFA substantially increased tissue concentration of EPA and DHA, and decreased macrophage infiltration and thickened the fibrous cap in the human carotid artery [111].
This entry is adapted from the peer-reviewed paper 10.3390/ijms20164025
This entry is offline, you can click here to edit this entry!
