Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biotechnology & Applied Microbiology
This topic discusses the properties of chitosan, which is synthesized from the biopolymer named chitin. Chitin is available in nature in abundance. Chitosan is a biodegradable polymer with other biological properties like antioxidant and antimicrobial properties. The polymer and its derivatives are nonimmunogenic and noninflammatory. Due to their advantages, these polymers have received much attention in the food and pharmaceutical industries. In the present topic, the sources of chitosan and their properties will be discussed in brief.
- chitosan
- food
1. Properties of Chitosan
The food processing industries that deal with the different kinds of seafood generate many waste products, e.g., skins, shells, scales, internal tissues, etc. The weight of these wastes can be as high as >70% of the seafood source animal or organisms. The improper disposal (e.g., landfill, dumping in the sea/local water bodies, or burning) of these wastes can lead to increased environmental hazards. Hence, researchers have proposed the valorization of the waste products to the extent possible, which can reduce the burden on the environment to a great extent. The food industries dealing with crustaceans have generated ~75% of waste products [1]. The waste products of the crustacean industry are rich in proteins and chitin (a naturally occurring polysaccharide). The polysaccharide can also be extracted from the biomass of fungi, fly larvae, annelids, and yeasts (Figure 1) [2][1][3]. The natural abundance of chitin is second-most, after the cellulose. However, chitin is the most abundant polymer of natural origin [1]. Chitin is an amino polysaccharide. Chemically, chitin is composed of β-1,4 linked 2-(acetylamino)-2-deoxy-d-glucose elements (Figure 2) [1][3][4]. The degree of acetylation is >90%. The overall nitrogen content in chitin is ~7%, while the nitrogen to carbon (N/C) ratio is 0.146. This naturally occurring polymer is biodegradable, biocompatible, and has been explored for several food applications [3]. Unlike other polysaccharides (e.g., alginate, tamarind gum, guar gum, etc.), chitin is insoluble in traditional solvents, limiting its applications [3]. Due to this reason, researchers have proposed converting chitin to chitosan (Figure 3). This can be achieved by the controlled deacetylation of chitin [2]. The deacetylation of chitin is mainly carried out by two methods: the alkaline method and the enzymatic method. The alkaline method of deacetylation is carried out by refluxing chitin in 40–50% of sodium hydroxide solution for a specific period [5]. Unfortunately, this method is not environmentally friendly. The enzymatic method involves treating the chitin with a chitin deacetylase enzyme extracted from various bacterial strains, which is preferred to deacetylation, due to its eco-friendly nature [5]. Inside the chitin backbone, the deacetylation partially eliminates the acetyl groups from the acetylamino groups. This results in the conversion of the constituent elements of chitin to β-1,4 linked 2-(amino)-2-deoxy-d-glucose elements (Figure 2) [1][4]. The 1C4 conformation of the linkages of monomer units, formed due to equatorial glycosidic linkages, restricts the movement of the monomers to a great extent. This phenomenon makes the chitosan a stiff molecule, explaining the high intrinsic viscosity of chitosan solutions [1]. The free amino groups are mainly responsible for the chemical and functional properties of chitosan. Typically, the degree of acetylation in chitosan is lower than 40%. The deacetylation process does not eliminate the nitrogen from the polysaccharide. Hence, the nitrogen content of chitosan is always >7% [1].
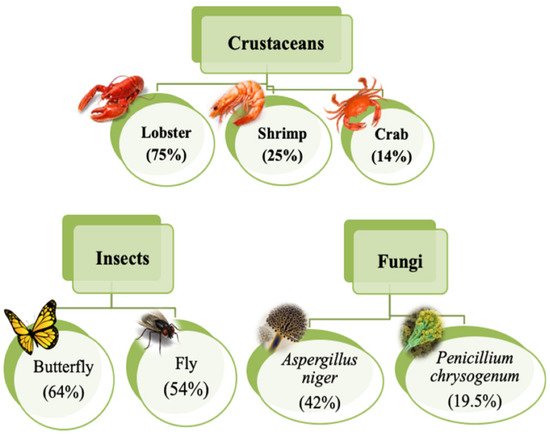
Figure 1. Different sources of chitin (taken from [6] under Creative Commons License).
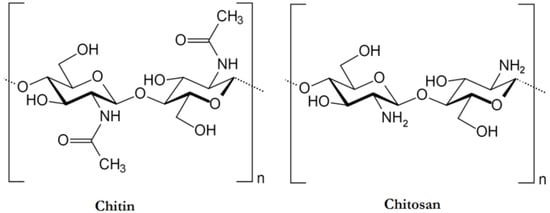
Figure 2. The chemical architecture of chitin and chitosan (taken from [4] under Creative Commons License).
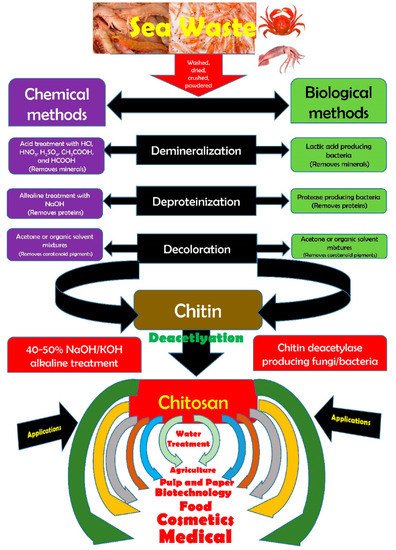
Figure 3. Modalities for the synthesis of chitosan and its applications (taken from [5] under Creative Commons License).
The solubility of chitosan in water is a major challenge for food scientists. It is important to note that the presence of a free amino group makes chitosan a basic polysaccharide. Due to this reason, the chitosan is solubilized at low pH solutions (usually below the pH of 6) [1]. However, some chitosans with a comparatively higher degree of acetylation (50%) solubilize at a pH of 7 [1]. Usually, chitosan has been reported to be solubilized in acidic solutions (e.g., hydrochloric acid, lactic acid, and acetic acid, etc.). Chitosan can be solubilized in weak acids, such as lactic acid and acetic acid, for food applications. Further, few researchers have reported converting chitosans into salts (e.g., chitosan lactate and chitosan glutamate) and derivatives (e.g., carboxymethyl chitosan). The conversion of chitosan into chitosan oligosaccharide, a low molecular weight chitosan product, which is soluble in water, has also been proposed. Chitosan and its derivatives have received much attention in the food industry, mainly for its antimicrobial and antioxidant properties (Figure 3). The polysaccharide has also been explored due to its viscosity-enhancing properties as a stabilizing agent, emulsifier, and controlled flavor-release matrices. The chitosan matrices have also been used to immobilize various food and nutraceutical agents, such as enzymes, vitamins, probiotics, etc. (Figure 4). Chitosan matrices have been used to immobilize the methanotrophs that resulted in improvised methanol production [7]. Since chitosan is a bulk-forming agent, it also serves as a source of dietary fibers for improving gut health.
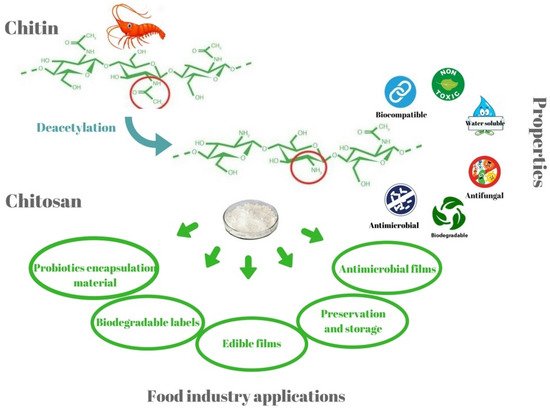
Figure 4. Some of the unique properties and food industry applications of chitosan (taken from [8] under Creative Commons License).
As reported in the previous paragraph, chitosan exhibits antimicrobial properties. This property allows the polymer to be used as a natural antimicrobial agent/preservative. Hence, the packaging materials developed with chitosan and its derivatives inherently exhibit antimicrobial properties towards a wide range of bacteria, fungus, and viruses. It is important to note that chitosan is biologically active against many microorganisms, including pathogenic microbes. Chitosan-based packaging systems have been found to prevent food products from Gram-positive and Gram-negative bacterial growth and fungal growth (Figure 5) [2]. The antimicrobial activity of chitosan has been related to the free amino group of the glucosamine residue. This fraction of chitosan can be easily protonated. The protonated amino groups then interact with the cell membranes of the microbes that are inherently anionic. The anionic behavior of the microbes can be explained due to the presence of phospholipids, lipopolysaccharides, peptides, and amino acids present on the surface of the microbial cell membranes [2]. The interaction between the cationic chitosan, or chitosan derivatives, and the anionic microbes, increases the permeability of the cell membranes and can disrupt the cell membranes. This results in the leakage of the internal cellular matter leading to the death of the microbes. Another plausible mechanism by which chitosan is expected to inhibit microbial cellular growth is the interaction of the chitosan molecules with the microbial cells’ genetic material (DNA or RNA). This inhibits DNA transcription, RNA translation, and protein synthesis [2]. A group of researchers also suggested that since chitosan is a cationic polyelectrolyte, it acts as a chelating agent for various ions, such as Cu2+, Hg2+, Zn2+, Cd2+, and Ni2+ [1]. Due to this reason, chitosan can interact with the essential nutrients and trace elements that are required for cell growth and toxin production by the pathogenic microbes, respectively [2]. Additionally, the chitosan molecules can also interact with the spore of the microbes and consequently neutralize them, which helps to prevent the growth of the microbes [2].
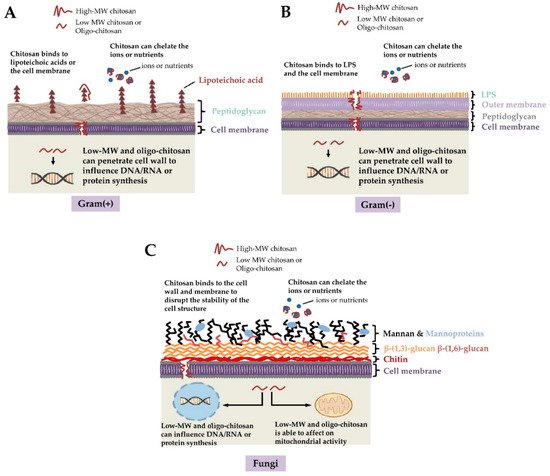
Figure 5. Mechanism of antimicrobial property exhibited by chitosan (taken from [9] under Creative Commons License).
The antioxidant property of the chitosan and its derivatives have been related to the free radical scavenging activity of the chitosan (Figure 6). Chitosan derives this property from the lone pair of electrons on the nitrogen atom located at the C-2 position. The lone pair of electrons allows the nitrogen atoms to accept a proton to form an ammonium (NH3+) group, which immediately reacts with the free radicals to generate stable molecules [10]. The antioxidant properties of chitosan and its derivatives have been extensively studied and documented using the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assays [11].
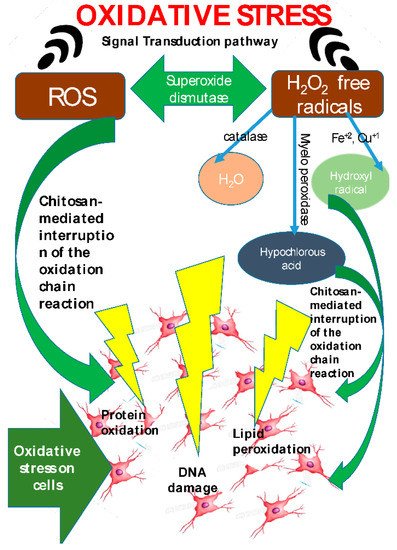
Figure 6. Mechanism of anti-oxidative property exhibited by chitosan (taken from [5] under Creative Commons License).
This entry is adapted from the peer-reviewed paper 10.3390/ijms222010968
References
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346.
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551.
- Wang, X.; Zhou, P.; Lv, X.; Liang, Y. Insight into the structure-function relationships of the solubility of chitin/chitosan in natural deep eutectic solvents. Mater. Today Commun. 2021, 27, 102374.
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174.
- Muthu, M.; Gopal, J.; Chun, S.; Devadoss, A.; Hasan, N.; Sivanesan, I. Crustacean waste-derived chitosan: Antioxidant properties and future perspective. Antioxidants 2021, 10, 228.
- da Silva Alves, D.C.; Healy, B.; Pinto, L.A.d.A.; Cadaval, T.R.S.; Breslin, C.B. Recent developments in chitosan-based adsorbents for the removal of pollutants from aqueous environments. Molecules 2021, 26, 594.
- Patel, S.K.; Gupta, R.K.; Kondaveeti, S.; Otari, S.V.; Kumar, A.; Kalia, V.C.; Lee, J.-K. Conversion of biogas to methanol by methanotrophs immobilized on chemically modified chitosan. Bioresour. Technol. 2020, 315, 123791.
- Călinoiu, L.-F.; Ştefănescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan coating applications in probiotic microencapsulation. Coatings 2019, 9, 194.
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial actions and applications of chitosan. Polymers 2021, 13, 904.
- Trung, T.S.; Bao, H.N.D. Physicochemical properties and antioxidant activity of chitin and chitosan prepared from Pacific white shrimp waste. Int. J. Carbohydr. Chem. 2015, 2015, 706259.
- Rajasree, S.R.; Gobalakrishnan, M.; Aranganathan, L.; Karthih, M. Fabrication and characterization of chitosan based collagen/ gelatin composite scaffolds from big eye snapper Priacanthus hamrur skin for antimicrobial and antioxidant applications. Mater. Sci. Eng. C 2020, 107, 110270.
This entry is offline, you can click here to edit this entry!
