Preeclampsia (PE) is a specific syndrome of human pregnancy, being one of the main causes of maternal death. Persistent inflammation in the endothelium stimulates the secretion of several inflammatory mediators, activating different signaling patterns. One of these mechanisms is related to NLRP3 activation, initiated by high levels of danger signals such as cholesterol, urate, and glucose, producing IL-1, IL-18, and cell death by pyroptosis. Furthermore, reactive oxygen species (ROS), act as an intermediate to activate NLRP3, contributing to subsequent inflammatory cascades and cell damage. Moreover, increased production of ROS may elevate nitric oxide (NO) catabolism and consequently decrease NO bioavailability. NO has many roles in immune responses, including the regulation of signaling cascades. At the site of inflammation, vascular endothelium is crucial in the regulation of systemic inflammation with important implications for homeostasis.
1. Introduction
Preeclampsia (PE) is a specific syndrome of human pregnancy, considered the main cause of morbidity and mortality in 2 to 8% of pregnancies worldwide [
1], and one of the main causes of maternal death. The clinical parameters that characterize this pathology are arterial hypertension and proteinuria from the twentieth week of pregnancy or in the first days after delivery. However, other maternal dysfunctions may also be related to PE, such as renal failure, liver involvement, neurological or hematological complications, uteroplacental dysfunction, or fetal growth restriction [
2,
3]. This pathology increases the risk of maternal and fetal mortality, through placental abruption, cerebrovascular events, organ failure, and disseminated intravascular coagulation [
4].
Endothelial cells have different functions during non-inflammatory conditions, such as maintaining blood fluidity, regulating blood flow, and maintaining leukocytes in a basal state circulating [
10]. In cases of infection or inflammation, these cells recognize danger signals and they act as active regulators of the inflammatory response [
10], and receptors in these cells help the response to a range of external signals [
11]. This meeting between endothelial cells and danger signals, such as ATP and high mobility group box 1 protein (HMGB1), can activate the NOD-like receptor family, pyrin domain-containing protein 3 (NLRP3) [
12,
13]. Activation of NLRP3 inflammasome in endothelial cells was already observed in animal models, and production of IL-1β by these cells has been shown to contribute to diverse pathological conditions [
14,
15].
Several recent studies in the literature have demonstrated that women with PE present a significantly higher expression of NLRP3, and related mediators such as caspase-1, IL-1, and IL-18 compared to normotensive healthy pregnant women [
16,
17,
18].
2. Preeclampsia and Endothelial Dysfunction
Endothelial dysfunction is the term used to describe an imbalance in these endothelial functions affecting vasoprotective homeostasis [20].
In normal pregnancies, the typical increase in blood volume is commonly compensated by a slight decrease in blood pressure. For a long time, this rise in blood pressure has been associated with reduced maternal vascular resistance [21,22]. However, in PE, the compensatory maternal vascular adaptations are insufficient, and it has been associated with systemic endothelial dysfunction [23,24,25,26]. In this syndrome, this characteristic is associated with the PE poorly perfused placenta, which releases proinflammatory and antiangiogenetic factors into maternal circulation [27,28]. This hypothesis has been reinforced by many studies so far. For example, Myers and colleagues demonstrated that healthy myometrium vessels incubated with plasma from PE pregnant women had reduced endothelium mediated vascular relaxation compared to those incubated with plasma from healthy pregnant women [29]. Plasma from women with PE can modify the endothelial function, altering the balance between vasoactive substances. Despite evidence showing the release of placental factors into maternal circulation could alter endothelial function, the exact mechanism is not fully understood. [30].
Until today, many studies demonstrated several alterations, both locally and in circulation, in multiple bioactive factors in PE. For example, the angiogenic balance disturbance has been described by decreases in pro-angiogenic vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) by the action of the placental soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng) [29,31,32,33]. Moreover, proinflammatory molecules such as tumor necrosis factor-α (TNF-α), endocan, interleukin-6 (IL-6), and IL-1β have also been reported to be altered in PE [34,35,36,37]. Altogether, these alterations lead to systemic endothelial dysfunction in PE, and it also is possible that there may be more mechanisms involved that were not discovered yet.
3. NLRP3 Inflammasome Activation and Regulation in Preeclampsia
3.1. Inflammasome Formation and the Role of NLRP3 in the Pathogenesis of PE
The immune response is divided into innate and adaptive immunity. Contact with pathogens or any danger signal activates the innate immune system, as the first line of defense. This process starts quickly as possible to protect the organism and to maintain homeostasis. The immune cells detect the signals from invaders, expressing molecules known as pathogen-associated molecular patterns (PAMPs). Besides that, these cells also identify molecules associated with inflammation and cell death, in cases of sterile inflammation, without any external microbial sign. These molecules associated with inflammation are named damage-associated molecular patterns (DAMPs). PAMPs and DAMPs are recognized by pattern recognition receptors (PRRs). Two of the most studied PRRs are Toll-like receptors (TLRs) and nucleotide-binding domain leucine-rich repeat-containing receptors (NLRs) [
41].
There are 22 recognized members of the NLR family, between them, NLRP3 (NOD-like receptor family, pyrin domain-containing protein 3) is the most studied and investigated, because this NLR forms complexes with other proteins, forming multimeric complexes, called inflammasomes [
42].
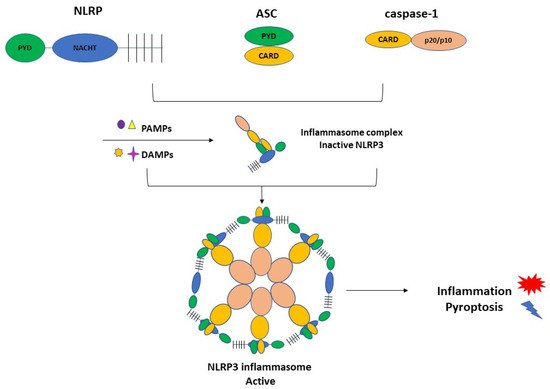
NLRP3 inflammasome complex is constituted by NLRP3, ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), and the cysteine protease precursor procaspase-1 (Figure 1).
Figure 1. The NLRP3 inflammasome consists of NLRP3, ASC, and caspase-1. NLRP3 is composed of C-terminal leucine-rich repeats (LRRs), a central nucleotide-binding and oligomerization domain (NACHT), and an N-terminal pyrin domain (PYD). ASC is also termed Pycard, containing an N-terminal PYD and a C-terminal caspase recruitment domain (CARD). The last element of the CARD and caspase domains. PAMPs and DAMPs can activate the inflammasome complex and triggers inflammation and pyroptosis.
Recently, reports regarding the NLRP3 inflammasome activation in PE have been increased. The literature shows higher expression of the NLRP3 inflammasome components in blood cells and placenta from PE women compared with normotensive healthy pregnant women [
16,
43,
44]. Furthermore, trophoblastic cells also express NLRP3, ASC, and caspase-1 [
45,
46,
47], and IL-1β secretion occurs in human trophoblast cells in response to activators of the NLRP3 inflammasome [
46,
47]. The interaction between alarmin-induced activation of placental NLRP3 inflammasome and the resulting placental inflammation presented in pregnancy complications such as preeclampsia has been shown by in vivo studies [
46,
48,
49,
50].
These recent contributions suggest that NLRP3 inflammasome activation is implicated in the inflammatory processes associated with the pathophysiology of preeclampsia. Moreover, in vitro and in vivo studies have shown that inflammatory stimuli induce the activation of the NLRP3 inflammasome in the placenta, also contributing to other pregnancy-related disorders [
51].
3.2. Activation of NLRP3 and Pyroptosis: The Cell Death Related to Inflammatory Processes
The literature data showed significantly higher expression of the NLRP3 and related mediators such as caspase-1, IL-1, and IL-18 in samples from women with PE compared to controls [
16,
44]. Other groups highlighted the NLRP3 gene polymorphisms associated with a significantly higher risk of disease development [
17,
18].
Inflammasome activation starts with two signals, both initiated by DAMPs or PAMPs [
52,
53].
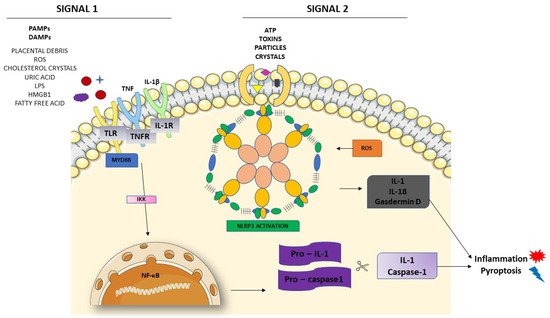
Figure 2 shows these two different signals in the activation of NLRP3. The first one is the priming signal, leading nuclear factor kappa B (NF-κB) activation through membrane receptors. NF-κB is important in the activation of the transcription and regulators of several genes, inducing the expression of pro-IL-1 and NLRP3. In the second signal, PAMPs and DAMPs appear to bind directly to NLRP3 [
53]. Once activated, NLRP3 interacts with ASC, recruiting and activating procaspase-1. The interaction between NLRP3 and ASC activates caspase-1, as well as pro-IL-1 and IL-18, releasing these cytokines in their active forms.
Figure 2. NLRP3 inflammasome activation. The priming signal (signal 1) occurs in the presence of danger signals (PAMPs and DAMPs), leading to the activation of the NF-κB and subsequent upregulation of NLRP3 and pro-IL-1 and pro-caspase1. The activation signal (signal 2) starts with the direct activation of the NLRP3 inflammasome with ROS recruitment. The process leads to inflammation and pyroptosis.
4. NLRP3 and its Relation with Endothelial Dysfunction and Oxidative Stress
Generally, DAMPs can trigger NLRP3 inflammasome activation, producing mature forms of IL-1β and IL-18 from cells to promote further inflammatory processes and oxidative stress in the endothelium [
62]. Endothelial cells (ECs) are a target of IL-1β, and it also produces IL-1β during inflammation [
63], activating other inflammatory mediators, contributing to secreting adhesion molecules and chemokines in ECs, inducing a potent pro-inflammatory response [
64]. Endothelial inflammation may initiate the occurrence and progression of endothelial dysfunction.
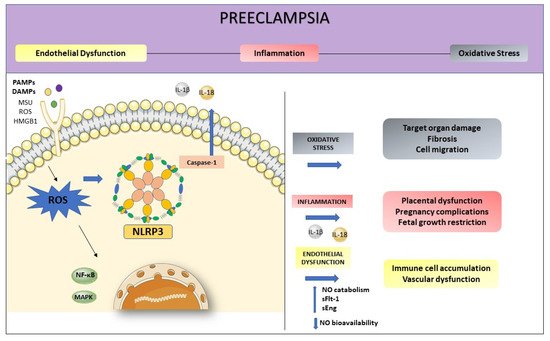
Oxidative stress and inflammation are inseparable events in inflammatory diseases and both play an essential role in the pathogenesis of PE (
Figure 3). The NLRP3 activation initiates from various stimuli, including the production of reactive oxygen species (ROS) [
65]. They are the first intermediate reactive products generated during inflammasome activation, being responsible for the release of inflammatory agents in the immune response [
66].
Figure 3. Preeclampsia is characterized by intense oxidative stress, inflammation, and endothelial dysfunction. The activation of NLRP3 may start with the production of ROS. Inflammasome activation is responsible for the release of inflammatory agents during the immune response, such as IL-1β and IL-18. High levels of ROS increase NO catabolism and decrease NO bioavailability as well as increasing factors such as sFlt-1 and sEng. This process enhanced inflammation-related genes expression, contributing to endothelial dysfunction.
In this way, ROS mediate the interaction between NLRP3 inflammasome and endothelial dysfunction, being the first participant in the NLRP3 activation, promoting inflammation, and activating immune responses [
66]. Three important proteins, thioredoxin-interacting protein (TXNIP), nuclear factor kappaB (NF-κB), and the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) are involved in the oxidative stress, connecting ROS to NLRP3 activation [
67]. In a state of increased oxidative stress, as occurs in preeclampsia, the imbalance between pro and antioxidants, coupled with higher ROS production may increase NO catabolism, and decrease NO bioavailability. The oxidative stress enhanced inflammation-related genes expression and increased inflammatory proteins, impairing endothelial function [
68].
5. Conclusions
NLRP3 activation plays an important role in the development of PE. Although NLRP3 has been the most intensively investigated type of inflammasome, a total mechanism for its activation has not yet been elucidated. Therefore, inhibitors of NLRP3 could be a very effective treatment for PE. With new research, the mechanisms regarding endothelial function and its relation to the NLRP3 inflammasome activation pathway can be better elucidated. Meanwhile, the interactions between endothelial dysfunction, oxidative stress, and the NLRP3 inflammasome-regulated pathways may improve the treatments of inflammation-related disorders, such as PE.
This entry is adapted from the peer-reviewed paper 10.3390/cells10112828