Toxoplasma gondii (T. gondii) is a prevalent protozoan parasite of medical and veterinary significance. It is the etiologic agent of toxoplasmosis, a neglected disease in which incidence and symptoms differ between patients and regions. In immunocompetent patients, toxoplasmosis manifests as acute and chronic forms. Acute toxoplasmosis presents as mild or asymptomatic disease that evolves, under the host immune response, into a persistent chronic disease in healthy individuals. Chronic toxoplasmosis establishes as latent tissue cysts in the brain and skeletal muscles. In immunocompromised patients, chronic toxoplasmosis may reactivate, leading to a potentially life-threatening condition.
- toxoplasmosis
- neuropathies
1. Introduction
2. Toxoplasma gondii Pathogenesis
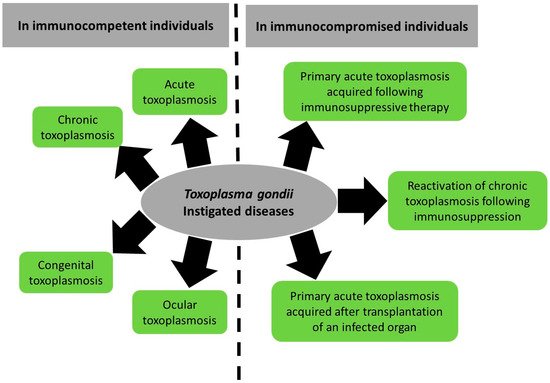
2.1. Toxoplasmosis in Immunocompetent Patients
2.1.1. Acute Toxoplasmosis
2.1.2. Congenital Toxoplasmosis
2.1.3. Ocular Toxoplasmosis
2.1.4. Chronic Toxoplasmosis
2.2. Toxoplasmosis in Immunocompromised Patients
3. Toxoplasma gondii-Associated Diseases
3.1. Toxoplasma gondii and Primary Neuropathies
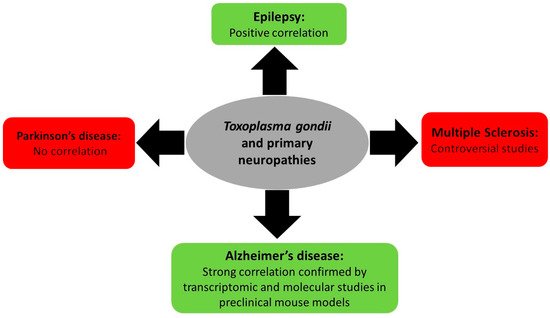
3.1.1. Toxoplasmosis and Multiple Sclerosis
3.1.2. Toxoplasmosis and Epilepsy
3.1.3. Toxoplasmosis and Parkinson’s and Alzheimer’s Neuropathies
3.2. Toxoplasma gondii, Psychiatric and Behavioral Disorders
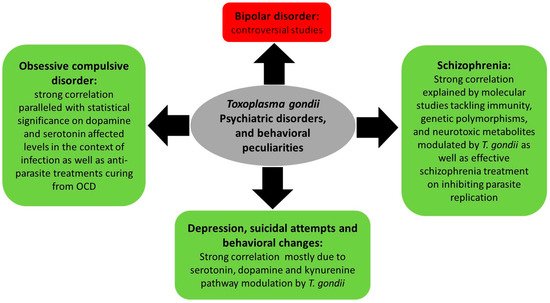
3.2.1. Toxoplasmosis, Depression, and Behavioral Changes
3.2.2. Toxoplasmosis and Schizophrenia
3.2.3. Toxoplasmosis and Bipolar Disorder
3.2.4. Toxoplasmosis and Obsessive Compulsive Disorder
3.3. Toxoplasma gondii and Cancers: Modulation of miRNAs as One Molecular Explanation of Toxoplasma-Associated Brain Cancers
This entry is adapted from the peer-reviewed paper 10.3390/pathogens10111351
