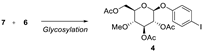
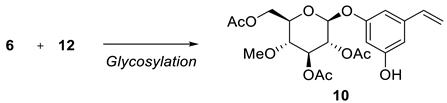
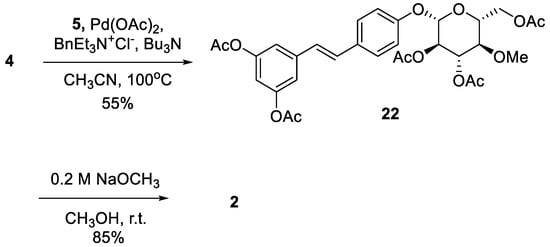
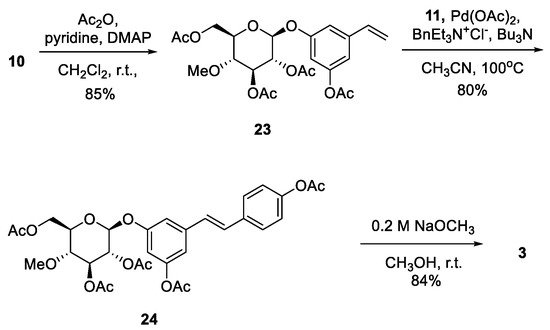
Resveratrol is a well-known dietary polyphenol because it has a variety of beneficial biological activities. The fungus Beauveria bassiana is one of the most frequently used microorganisms for the biotransformation of polyphenols. Recently, resvebassianol A (2), a glycosylated metabolite of resveratrol by B. bassiana, was isolated and structurally elucidated. It was demonstrated to exhibit antioxidant, regenerative, and anti-inflammatory activities with no cytotoxicity. Here, we report the first total synthesis of resvebassianol A, 4'-O-β-(4'"-O-methylglucopyranosyl)resveratrol (2), and its regiomer, 3-O-β-(4 -O-methylglucopyranosyl)resveratrol (3). Key reactions include (i) the construction of a stilbene core via a novel Heck reaction of aryl halides and styrenes, and (ii) glycosylation with unnatural methylglucopyranosyl bromide. The glycosylation step was carefully optimized by varying the bases and solvents. Resveratrol metabolites 2 and 3 were obtained at 7.5% and 6.3% of the overall yield, respectively.
- resveratrol
- resvebassianol A
- Beauveria bassiana
- metabolites
- glycoslyation
1. Introduction
2. Results and Discussion
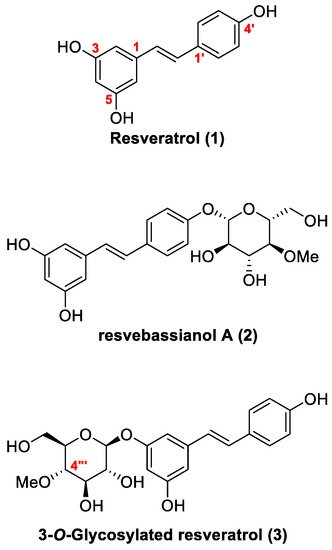
2.1. Retrosynthesis
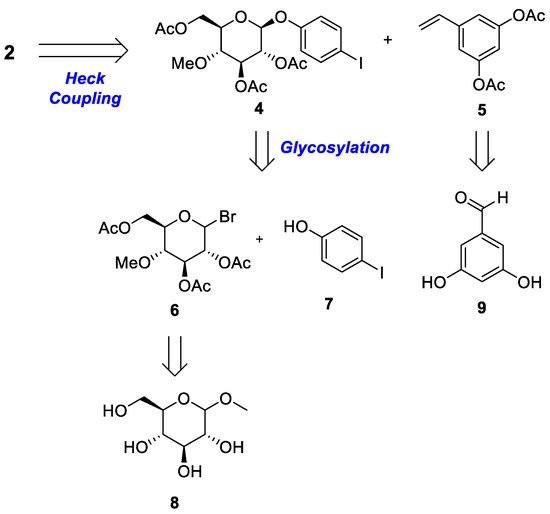
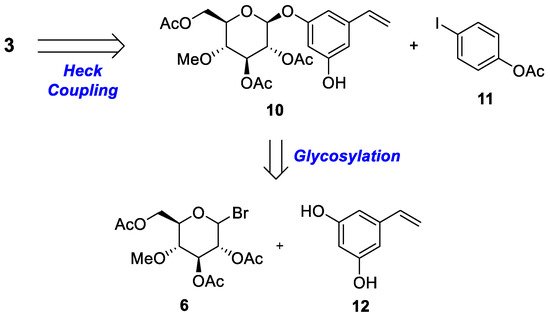
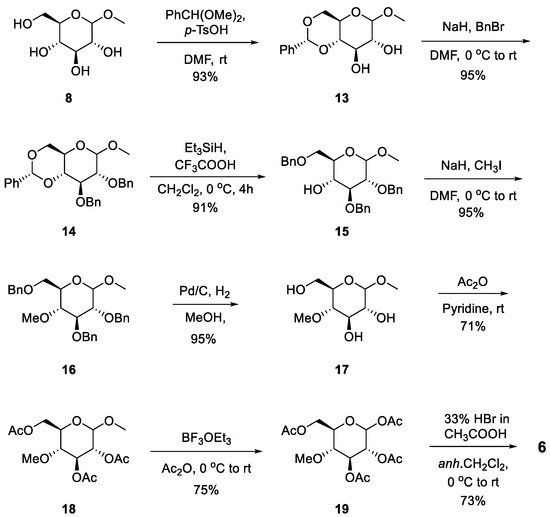
2.2. Chemistry

| Entry a | Base | Reagent | Solvent | Temp. (°C) | Yield (%) d |
|---|---|---|---|---|---|
| 1 | Ag2CO3 (1eq) | CH3CN | r.t | 16 | |
| 2 b | NaOH | TBAB | CHCl3:H2O (1:1) | 45 | 14 |
| 3 b | K2CO3 | TBAB | CHCl3:H2O (1:1) | 45 | 17 |
| 4 c | K2CO3 | BnNBu3Cl | CHCl3 | r.t | 57 |
| Entry | Base | Reagent | Solvent | Temp. (°C) | Yield (%) d |
|---|---|---|---|---|---|
| 1 | Ag2CO3 | CH3CN | r.t | 19 | |
| 2 b | NaOH | TBAB | CHCl3:H2O (1:1) | 45 | 13 |
| 3 b | K2CO3 | TBAB | CHCl3:H2O (1:1) | 45 | 15 |
| 4 c | K2CO3 | BnNBu3Cl | CHCl3 | r.t | 40 |
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/antiox10101509
References
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220.
- Wang, Y.; Catana, F.; Yang, Y.; Roderick, R.; van Breemen, R.B. An LC-MS Method for Analyzing Total Resveratrol in Grape Juice, Cranberry Juice, and in Wine. J. Agric. Food Chem. 2002, 50, 431–435.
- Lucas, R.; Alcantara, D.; Morales, J.C. A concise synthesis of glucuronide metabolites of urolithin-B, resveratrol, and hydroxytyrosol. Carbohydr. Res. 2009, 344, 1340–1346.
- Fritzemeier, K.-H.; Kindl, H. Coordinate induction by UV light of stilbene synthase, phenylalanine ammonia-lyase and cinnamate 4-hydroxylase in leaves of vitaceae. Planta 1981, 151, 48–52.
- Schultz, T.P.; Boldin, W.D.; Fisher, T.H.; Nicholas, D.D.; Mcmurtrey, K.D.; Pobanz, K. Structure-fungicidal properties of some 3- and 4-hydroxylated stilbenes and bibenzyl analogues. Phytochemistry 1992, 31, 3801–3806.
- Takaoka, M. Of the phenolic substrate of hellebore (Veratrum grandiflorum Loes. fil.). J. Fac. Sci. Hokkaido Imper. Univ. 1940, 3, 1–16.
- Quideau, S.; Deffieux, D.; Pouységu, L. Resveratrol Still Has Something To Say about Aging! Angew. Chem. Int. Ed. 2012, 51, 6824–6826.
- Gülçin, I. Antioxidant properties of resveratrol: A structure—Activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218.
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723.
- Lançon, A.; Frazzi, R.; Latruffe, N. Anti-Oxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Resveratrol in Ocular Diseases. Molecules 2016, 21, 304.
- Raval, A.P.; Lin, H.W.; Dave, K.R.; DeFazio, R.A.; Morte, D.; Kim, E.J.; Perez-Pinzon, M.A. Resveratrol and Ischemic Preconditioning in the Brain. Curr. Med. Chem. 2008, 15, 1545–1551.
- Cho, S.; Namkoong, K.; Shin, M.; Park, J.; Yang, E.; Ihm, J.; Thu, V.T.; Kim, H.K.; Han, J. Cardiovascular Protective Effects and Clinical Applications of Resveratrol. J. Med. Food 2017, 20, 323–334.
- Yang, X.; Xu, S.; Qian, Y.; Xiao, Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav. Immun. 2017, 64, 162–172.
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1–10.
- Li, Y.-R.; Li, S.; Lin, C.-C. Effect of resveratrol and pterostilbene on aging and longevity. BioFactors 2018, 44, 69–82.
- Kapadia, G.J.; Azuine, M.A.; Tokuda, H.; Takasaki, M.; Mukainaka, T.; Konoshima, T.; Nishino, H. Chemopreventive effect of resveratrol, sesamol, sesame oil and sunflower oil in the Epstein-Barr virus early antigen activation assay and the mouse skin two-stage carcinogenesis. Pharmacol. Res. 2002, 45, 499–505.
- Szkudelska, K.; Nogowski, L.; Szkudelski, T. Resveratrol, a naturally occurring diphenolic compound, affects lipogenesis, lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J. Steroid Biochem. Mol. Biol. 2009, 113, 17–24.
- Dermani, F.K.; Saidijam, M.; Amini, R.; Mahdavinezhad, A.; Heydari, K.; Najafi, R. Resveratrol Inhibits Proliferation, Invasion, and Epithelial-Mesenchymal Transition by Increasing miR-200c Expression in HCT-116 Colorectal Cancer Cells. J. Cell. Biochem. 2017, 118, 1547–1555.
- Porcu, M.; Chiarugi, A. The emerging therapeutic potential of sirtuin-interacting drugs: From cell death to lifespan extension. Trends Pharmacol. Sci. 2005, 26, 94–103.
- Shakibaei, M.; Harikumar, K.B.; Aggarwal, B.B. Resveratrol addiction: To die or not to die. Mol. Nutr. Food Res. 2009, 53, 115–128.
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506.
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15.
- Shimoda, K.; Kubota, N.; Uesugi, D.; Hamada, H.; Tanigawa, M.; Hamada, H. Synthesis and pharmacological evaluation of glycosides of resveratrol, pterostilbene, and piceatannol. Ann. N. Y. Acad. Sci. 2015, 1348, 141–149.
- Biasutto, L.; Marotta, E.; Bradaschia, A.; Fallica, M.; Mattarei, A.; Garbisa, S.; Zoratti, M.; Paradisi, C. Soluble polyphenols: Synthesis and bioavailability of 3,4′,5-tri(α-d-glucose-3-O-succinyl) resveratrol. Bioorganic Med. Chem. Lett. 2009, 19, 6721–6724.
- Regev-Shoshani, G.; Shoseyov, O.; Bilkis, I.; Kerem, Z. Glycosylation of resveratrol protects it from enzymic oxidation. Biochem. J. 2003, 374, 157–163.
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 5. Biologically active glycosides of aromatic metabolites. Lipids 2005, 40, 869–900.
- Torres, P.; Poveda, A.; Jimenez-Barbero, J.; Parra, J.L.; Comelles, F.; Ballesteros, A.O.; Plou, F.J. Enzymatic Synthesis of α-Glucosides of Resveratrol with Surfactant Activity. Adv. Synth. Catal. 2011, 353, 1077–1086.
- Cichewicz, R.H.; Kouzi, S.A. Biotransformation of Resveratrol to Piceid byBacillus cereus. J. Nat. Prod. 1998, 61, 1313–1314.
- Holland, H.L.; Morris, T.A.; Nava, P.J.; Zabic, M. A new paradigm for biohydroxylation by Beauveria bassiana ATCC 7159. Tetrahedron 1999, 55, 7441–7460.
- Zhan, J.; Gunatilaka, A.A.L. Selective 4′-O-methylglycosylation of the pentahydroxy-flavonoid quercetin byBeauveria bassianaATCC 7159. Biocatal. Biotransform. 2006, 24, 396–399.
- Ha, S.K.; Kang, M.C.; Lee, S.; Darlami, O.; Shin, D.; Choi, I.; Kim, K.H.; Kim, S.Y. Generation of Stilbene Glycoside with Promising Cell Rejuvenation Activity through Biotransformation by the Entomopathogenic Fungus Beauveria Bassiana. Biomedicines 2021, 9, 555.
- Wang, L.-X.; Heredia, A.; Song, H.; Zhang, Z.; Yu, B.; Davis, C.; Redfield, R. Resveratrol glucuronides as the metabolites of resveratrol in humans: Characterization, synthesis, and anti-HIV activity. J. Pharm. Sci. 2004, 93, 2448–2457.
- Learmonth, D.A. A Concise Synthesis of the 3-O-β-D- and 4‘-O-β-D-Glucuronide Conjugates of trans-Resveratrol. Bioconjugate Chem. 2003, 14, 262–267.
- Botella, L.; Nájera, C. Synthesis of methylated resveratrol and analogues by Heck reactions in organic and aqueous solvents. Tetrahedron 2004, 60, 5563–5570.
- DeNinno, M.P.; Etienne, J.B.; Duplantier, K.C. A method for the selective reduction of carbohydrate 4,6-O-benzylidene acetals. Tetrahedron Lett. 1995, 36, 669–672.
- Montero, J.-L.; Winum, J.-Y.; Leydet, A.; Kamal, M.; Pavia, A.A.; Roque, J.-P. A convenient synthesis of peracetylated glycosyl halides using bismuth(III) halides as catalysts. Carbohydr. Res. 1997, 297, 175–180.
- Hongu, M.; Saito, K.; Tsujihara, K. Solid-Liquid Phase Transfer Catalyzed Novel Glycosylation Reaction of Phenols. Synth. Commun. 1999, 29, 2775–2781.
