Adenoviruses represent exceptional candidates for wide-ranging therapeutic applications, from vectors for gene therapy to oncolytics for cancer treatments. The first ever commercial gene therapy medicine was based on a recombinant adenovirus vector, while most recently, adenoviral vectors have proven critical as vaccine platforms in effectively controlling the global coronavirus pandemic.
- adenovirus
- endocytosis
- vectors
- gene therapy
1. Introduction
We distinguish the two types of commonly used adenoviral vectors: replication-defective and oncolytic adenoviruses. Due to molecular engineering, replication-defective AdVs cannot replicate in infected cells, however they provide high expression of transgenes with minimum expression of AdV proteins. Unlike replication-defective AdVs (also known as vectors), oncolytic AdVs selectively replicate in and lyse cancer cells, and are, therefore, commonly used vectors in clinical trials for cancer gene therapy [6]. The world first oncolytic virus product was also an adenovirus, Oncorine (H101), an oncolytic adenovirus intended to be used in combination with chemotherapy as a treatment for patients with late-stage refractory nasopharyngeal cancer [7]. Currently, a large number of AdV platforms are being developed for oncology applications, for example, Enadenotucirev, a clinical stage oncolytic AdV with broad potential to target solid tumor carcinomas [8]; DNX-2401; Delta-24-RGD oncolytic AdV, developed as immunotherapeutic treatment for recurrent malignant glioma [9]; and TILT-123, a human AdV5/AdV3 chimeric oncolytic adenovirus encoding for tumor necrosis factor alpha and human interleukin 2 aimed at generating an anti-cancer immune response [10].
To fulfil its role as a vector, an AdV needs to successfully deliver its DNA genome to the host nucleus, a process highly influenced by AdV intracellular translocation. In this review, we discuss which factors are involved in AdV endocytosis, focusing mainly on receptors and endocytic scaffold proteins, as well as the influence of a particular type of endocytosis on AdV intracellular trafficking.
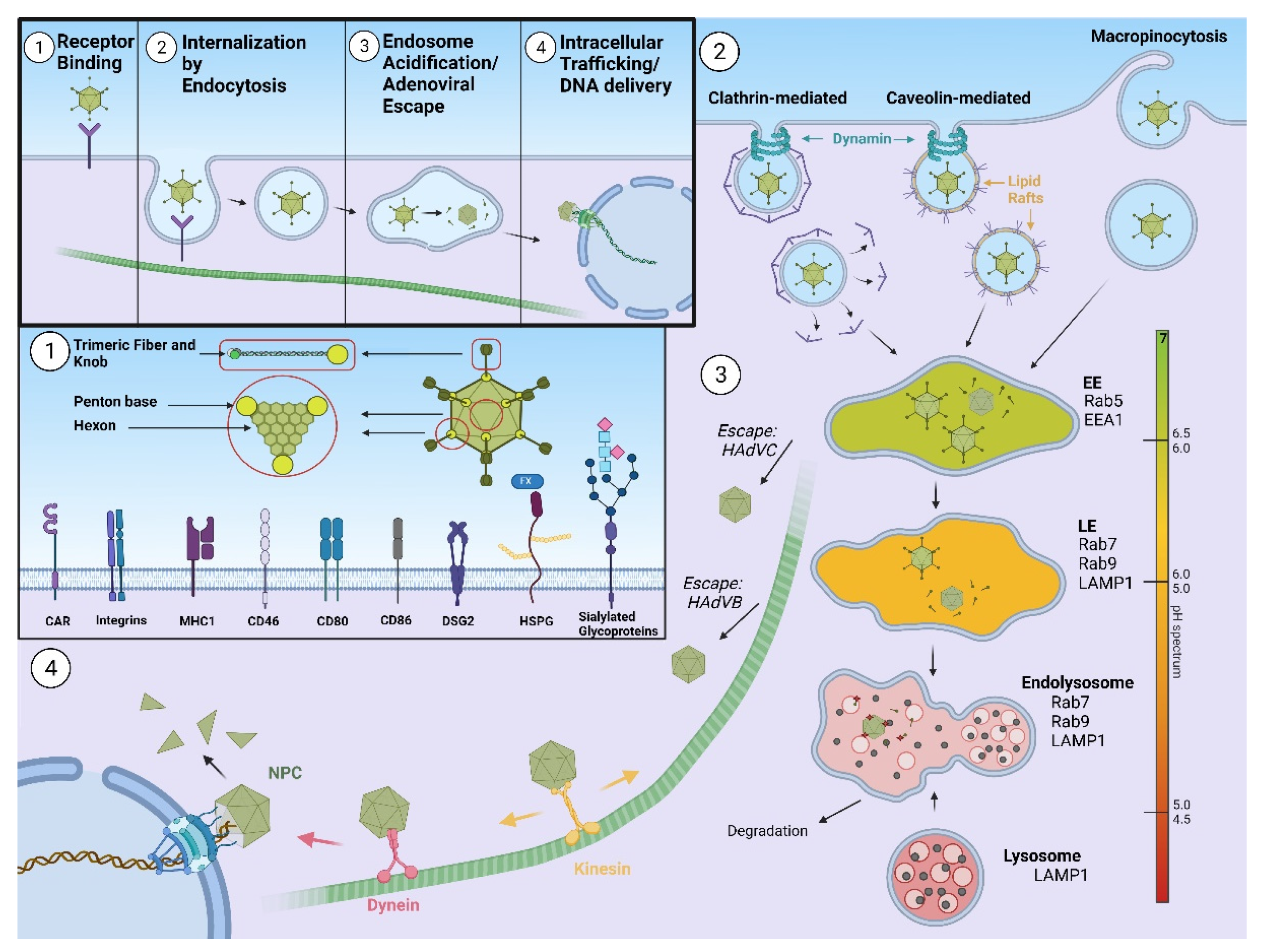
2. Host Innate Immune Response and Human Adenovirus Escape from Endosome
3. Outlook
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13101585
References
- Ghebremedhin, B. Human adenovirus: Viral pathogen with increasing importance. Eur. J. Microbiol. Immunol. 2014, 4, 26–33.
- Peng, Z. Current status of gendicine in China: Recombinant human Ad-p53 agent for treatment of cancers. Hum. Gene Ther. 2005, 16, 1016–1027.
- Pollard, A.J.; Launay, O.; Lelievre, J.D.; Lacabaratz, C.; Grande, S.; Goldstein, N.; Robinson, C.; Gaddah, A.; Bockstal, V.; Wiedemann, A.; et al. Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): A randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2021, 21, 493–506.
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111.
- Sadoff, J.; Gray, G. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201.
- Cunliffe, T.G.; Bates, E.A.; Parker, A.L. Hitting the Target but Missing the Point: Recent Progress towards Adenovirus-Based Precision Virotherapies. Cancers 2020, 12, 3327.
- Liang, M. Oncorine, the World First Oncolytic Virus Medicine and its Update in China. Curr. Cancer Drug Targets 2018, 18, 171–176.
- Illingworth, S.; Di, Y.; Bauzon, M.; Lei, J.; Duffy, M.R.; Alvis, S.; Champion, B.; Lieber, A.; Hermiston, T.; Seymour, L.W.; et al. Preclinical Safety Studies of Enadenotucirev, a Chimeric Group B Human-Specific Oncolytic Adenovirus. Mol. Ther. Oncolytics 2017, 5, 62–74.
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1419–1427.
- Havunen, R.; Siurala, M.; Sorsa, S.; Grönberg-Vähä-Koskela, S.; Behr, M.; Tähtinen, S.; Santos, J.M.; Karell, P.; Rusanen, J.; Nettelbeck, D.M.; et al. Oncolytic Adenoviruses Armed with Tumor Necrosis Factor Alpha and Interleukin-2 Enable Successful Adoptive Cell Therapy. Mol. Ther. Oncolytics 2017, 4, 77–86.
- Mangel, W.F.; San Martín, C. Structure, function and dynamics in adenovirus maturation. Viruses 2014, 6, 4536–4570.
- Nemerow, G.R.; Stewart, P.L.; Reddy, V.S. Structure of human adenovirus. Curr. Opin. Virol. 2012, 2, 115–121.
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717.
- Stewart, P.L.; Nemerow, G.R. Cell integrins: Commonly used receptors for diverse viral pathogens. Trends Microbiol. 2007, 15, 500–507.
- Greber, U.F.; Willetts, M.; Webster, P.; Helenius, A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 1993, 75, 477–486.
- Wiethoff, C.M.; Wodrich, H.; Gerace, L.; Nemerow, G.R. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 2005, 79, 1992–2000.
- Suomalainen, M.; Luisoni, S.; Boucke, K.; Bianchi, S.; Engel, D.A.; Greber, U.F. A direct and versatile assay measuring membrane penetration of adenovirus in single cells. J. Virol. 2013, 87, 12367–12379.
- Suomalainen, M.; Nakano, M.Y.; Keller, S.; Boucke, K.; Stidwill, R.P.; Greber, U.F. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J. Cell Biol. 1999, 144, 657–672.
- Scherer, J.; Vallee, R.B. Adenovirus recruits dynein by an evolutionary novel mechanism involving direct binding to pH-primed hexon. Viruses 2011, 3, 1417–1431.
- Zhou, J.; Scherer, J. Role of kinesins in directed adenovirus transport and cytoplasmic exploration. PLoS Pathog. 2018, 14, e1007055.
- Strunze, S.; Engelke, M.F.; Wang, I.H.; Puntener, D.; Boucke, K.; Schleich, S.; Way, M.; Schoenenberger, P.; Burckhardt, C.J.; Greber, U.F. Kinesin-1-mediated capsid disassembly and disruption of the nuclear pore complex promote virus infection. Cell Host Microbe 2011, 10, 210–223.
- Hendrickx, R.; Stichling, N.; Koelen, J.; Kuryk, L.; Lipiec, A.; Greber, U.F. Innate immunity to adenovirus. Hum. Gene Ther. 2014, 25, 265–284.
- Iacobelli-Martinez, M.; Nemerow, G.R. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J. Virol. 2007, 81, 1305–1312.
- Barlan, A.U.; Griffin, T.M.; McGuire, K.A.; Wiethoff, C.M. Adenovirus membrane penetration activates the NLRP3 inflammasome. J. Virol. 2011, 85, 146–155.
- Barlan, A.U.; Danthi, P.; Wiethoff, C.M. Lysosomal localization and mechanism of membrane penetration influence nonenveloped virus activation of the NLRP3 inflammasome. Virology 2011, 412, 306–314.
- Lee, J.S.; Mukherjee, S.; Lee, J.Y.; Saha, A.; Chodosh, J.; Painter, D.F.; Rajaiya, J. Entry of Epidemic Keratoconjunctivitis-Associated Human Adenovirus Type 37 in Human Corneal Epithelial Cells. Invest. Ophthalmol. Vis. Sci. 2020, 61, 50.
- Yousuf, M.A.; Zhou, X.; Mukherjee, S.; Chintakuntlawar, A.V.; Lee, J.Y.; Ramke, M.; Chodosh, J.; Rajaiya, J. Caveolin-1 associated adenovirus entry into human corneal cells. PLoS ONE 2013, 8, e77462.
- Lee, J.S.; Ismail, A.M.; Lee, J.Y.; Zhou, X.; Materne, E.C.; Chodosh, J.; Rajaiya, J. Impact of dynamin 2 on adenovirus nuclear entry. Virology 2019, 529, 43–56.
- Custers, J.; Kim, D.; Leyssen, M.; Gurwith, M.; Tomaka, F.; Robertson, J.; Heijnen, E.; Condit, R.; Shukarev, G.; Heerwegh, D.; et al. Vaccines based on replication incompetent Ad26 viral vectors: Standardized template with key considerations for a risk/benefit assessment. Vaccine 2021, 39, 3081–3101.
- Zahn, R.; Gillisen, G.; Roos, A.; Koning, M.; van der Helm, E.; Spek, D.; Weijtens, M.; Grazia Pau, M.; Radošević, K.; Weverling, G.J.; et al. Ad35 and ad26 vaccine vectors induce potent and cross-reactive antibody and T-cell responses to multiple filovirus species. PLoS ONE 2012, 7, e44115.
- Iacobelli-Martinez, M.; Nepomuceno, R.R.; Connolly, J.; Nemerow, G.R. CD46-utilizing adenoviruses inhibit C/EBPbeta-dependent expression of proinflammatory cytokines. J. Virol. 2005, 79, 11259–11268.
- Teigler, J.E.; Kagan, J.C.; Barouch, D.H. Late endosomal trafficking of alternative serotype adenovirus vaccine vectors augments antiviral innate immunity. J. Virol. 2014, 88, 10354–10363.
- Greber, U.F.; Webster, P.; Weber, J.; Helenius, A. The role of the adenovirus protease on virus entry into cells. EMBO J. 1996, 15, 1766–1777.
- Smith, J.S.; Xu, Z.; Tian, J.; Palmer, D.J.; Ng, P.; Byrnes, A.P. The role of endosomal escape and mitogen-activated protein kinases in adenoviral activation of the innate immune response. PLoS ONE 2011, 6, e26755.
- Zaiss, A.K.; Vilaysane, A.; Cotter, M.J.; Clark, S.A.; Meijndert, H.C.; Colarusso, P.; Yates, R.M.; Petrilli, V.; Tschopp, J.; Muruve, D.A. Antiviral Antibodies Target Adenovirus to Phagolysosomes and Amplify the Innate Immune Response. J. Immunol. 2009, 182, 7058.
