Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Plant heat shock proteins (HSPs), as chaperones, play a pivotal role in conferring biotic and abiotic stress tolerance. Moreover, HSP also enhances membrane stability and detoxifies the reactive oxygen species (ROS) by positively regulating the antioxidant enzymes system.
- heat shock factor
- biotic stress
- abiotic stress
- protein folding
- stress resistance
1. Introduction
Plants are sessile organisms and are subjected to various threats, both biotic and abiotic. These stresses, individually or in combination, result in huge losses in terms of growth, development, and yield and sometimes threaten the survival of the plant [1]. Plants continuously confront harsh environments like high/low temperatures, drought, salts, heavy metals, light, flooding and physical wounding [2,3,4,5]. Biotic stresses like pathogens (Viruses, Bacteria, Fungi) and pests such as nematodes, insects, and rodents also restrict plant productivity [6,7,8,9,10]. Negative effects of these stresses on the plant germination [11], are stunted growth [12,13], sunburn and scorching of leaves [14], loss of photosynthetic pigment, decreased production of photo-assimilates, and depletion of carbohydrate reserves which results in starvation [15,16,17,18]. Abiotic stresses also negatively affect the reproductive characteristics of plants by enhancing male sterility [19] and increasing premature flower and fruit drop [20] which results in significant low yield and quality. It has been reported that the increase in temperature by 1 °C results in a 4–10% yield decrease [21]. As a consequence of these stresses, reactive oxygen species (ROS) are produced which lead to oxidative stress and, ultimately, results in cell death. ROS could be singlet oxygen (1O2), superoxide radical (O2•−), hydrogen peroxide (H2O2) and hydroxyl radical (OH −), which are produced in cell organelles such as mitochondria, peroxisomes and chloroplasts in oxidative stress situations and react with all types of macromolecules like pigments, proteins, lipids and DNA [22,23].
Plants respond morphologically to elevated temperature and light stress by changing the leaf orientation [13], anatomically by altering stomatal conductance and increased leaf pubescence [24,25], and phenologically by shifting and improvising the developmental stages to escape the abiotic stress condition [26]. Plants also change the metabolic processes and physiology to retain root hydraulic conductance [27], accumulation of the compatible osmolytes, such as sugars, sugar alcohols, proline and phenolic compounds under saline and water-logged conditions, as well as high temperature and water deficit conditions [28]. Moreover, plants manage to maintain photosynthetic machinery [29] by changing their assimilate partitioning a shift occurs from symplastic to apo-plastic [30]. During the onset of the stress situations, plants also improvise the hormonal balance of abscisic acid (ABA), ethylene, and salicylic acid (SA) as a signaling molecule in the systemic acquired resistance. Similarly, jasmonic acid (JA) and other steroids enhance stress tolerance and resistance [31]. Furthermore, secondary metabolites, such as isopropanioid, carotenoid, flavonoid, anthocyanin, lignin, and isoprenoids [30,32], also are produced and accumulated.
Besides these adaptations, plants also have sophisticated adaptive systems at the cellular and molecular levels. During the onset of stress, plants reduce the synthesis of normal protein production, and transcribe and translate heat shock proteins (HSPs). Added to transcriptional regulations, plants also have some sophisticated post-transcriptional modifications which help the plant to cope with these stresses, such as alternative splicing and micro RNA (miRNA). Alternative splicing, which generates multiple copies from a single gene, helps the plants to mitigate abiotic stresses [33]. One of the important plant post-transcriptional modification strategies is the miRNA, which binds to the mRNA at any point to repress translation or direct cleavage of the mRNA. Some of the miRNAs also are involved with abiotic stress tolerance [34].
2. Occurrence of HSP in Plants
The heat shock response is not unique in plants. It was first discovered in the early 1960s by an Italian scientist, F. Ritossa, in Drosophila melanogaster, while working on high-temperature stress [58,59]. HSPs were studied in Saccharomyces cerevisiae, by McAlister et al. (1980) [60], Escherichia coli, by Yamamori et al. (1982) [61], and plants (Glycine max), Lin et al. (1984) [62]. A comparison of the response in different organisms has shown that HSPs are conserved highly across organisms [63]. The evolutionary conservation of the heat shock response shows that the production of HSPs is a fundamental and essential process in all organisms [51].
Plants, due to their sessile nature, have evolved different efficient strategies to ensure their generations in the challenging competitive environment. The numbers of stress related genes, including HSPs, are greater in plants than other organisms due to whole genome duplication and gene retention in their evolutionary past [64]. Additionally, plants contain chloroplasts which have their own genome and de novo protein synthesis machinery [65]. A strategy of the plant to cope with stressful situations (Figure 1), is the synthesis of normal protein reduced while the synthesis of stress-related proteins i.e., HSPs, is enhanced.
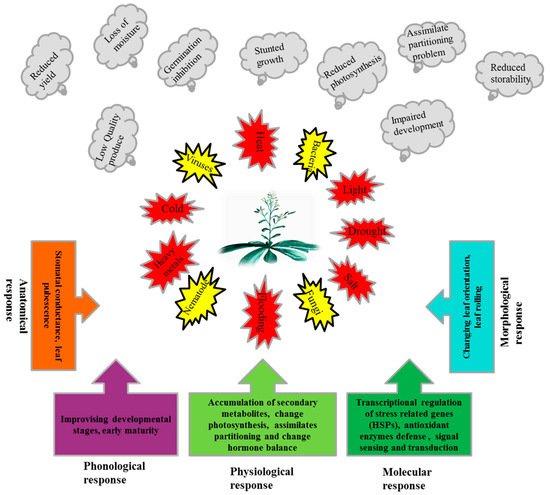
Figure 1. Exposures of plants to different biotic and abiotic stresses, adverse effects of these stresses on plants and response mechanisms of plants to these stresses.
Many HSPs have been reported in a wide range of organisms from prokaryotes to eukaryotes [63,66]. These HSPs across the organisms are conserved highly, with little difference except for HSP33, which differs in plants from that in bacteria [67]. The genes which encode different HSPs are found in different cell compartments, such as the nucleus, mitochondria, chloroplast, endoplasmic reticulum and cytosol [68]. Similarly, the accumulation of these HSPs in different parts of the cell also depends on the intensity of the stress. Nuclear HSPs, for instance, are accumulating in the cytosol at the lower and higher temperatures of 27 °C and 43 °C, respectively, while the same aggregate in chloroplast is at 37 °C [47]. Different HSPs are found and differentially expressed in different species, even in different genotypes but in the same species, as investigated by Korotaeva et al., (2001) and Nieto-Sotelo et al., (2002) [69,70] in small HSPs where five sHSPs showed response to a higher temperature (42 °C) in maize but only one expressed in wheat and rice. Likewise, HSP68 expressed in mitochondria under stress situations in potatoes, tomatoes, and soybeans [71]. Some HSPs showed a tissue-specific response to stress situations; HSP101 was expressed more in reproductive parts like tassels, ear, and endosperm, than in vegetative parts like leaves and roots in maize [72]. Some HSPs responded differently to the varying length of stresses. As Heckathorn et al., (1989) [73] reported, in HSP45, a nuclear protein accumulated in the chloroplast at a 3 h exposure to heat stress, which returned to its native state after removal of stress. Similarly, HSPs triggered differently with different development stages. HSP45, for example, showed a response in the whole plant to a stress situation, while HSP64 and -72 only showed expression in the reproductive parts i.e., pollens [74,75]. It can be deduced that these are the key regulators which show different responses to varying levels of stress in different parts of the plants.
3. Role of HSPs in Plant Defense
Plants are subjected to various biotic and abiotic stresses, singly or in combination, which adversely affect plant growth, development, and survival [86]. To cope with these stresses, plants have evolved various defense strategies i.e., physical [13], anatomical [25], and physiological [27,87]. Plants also respond to stress situations at the molecular level by altering gene expression, synthesis of stress-related biomolecules and proteins including heat stress proteins, to enable plants to ensure their generation in challenging situations.
3.1. Biotic Stress Tolerance
Plant growth, development, yield, and quality are affected adversely by several biotic factors such as pathogenic bacteria, fungi, viruses, and nematodes. Biotic factors, directly deprive their host plants of their nutrients, which result in reduced plant vigor, growth, productivity and sometimes leads to death of the host plants. Biotic stresses are major cause of pre- and post-harvest losses. Animals have an immune system, which helps them to adapt to biotic stresses such as new diseases and memorized the past infections, while plants lack such a system. Although plants lack this adaptive immune system, they have evolved several sophisticated strategies to counteract these biotic stresses [88]. These defense mechanisms are stored in the plant’s genome at the genetic level, which encode thousands of stress resistance genes. One of the adaptive systems plants employ in response to biotic stress is through regulation of HSPs (Table 2). HSP response to biotic stresses depend on the nature of the causal organisms and plant genotypes, either susceptible or resistant, and the developmental stage [6].
Table 2. Summary of the role of plant HSP under biotic stresses.
| Biotic Factor |
Plant | Pathogen | Disease Caused | HSP Response | Expression Pattern |
Reference |
|---|---|---|---|---|---|---|
| Viruses | Arabidopsis thaliana | TVCTV, ORTV, PVX, CMV, and TuMV |
Penstemon disease, red spots on leaves, deformed leaves and stunted growth | HSP17.6 HSP17.4 |
Up/down | [110] |
| Solanum lycopersicum | TYLCV | Stunted and bushy growth and excessive branches, abnormal leaf shapes, curled inward or upward, and flower and fruit drops | HSP70 HSP90 |
Up/down | [109,110,111] | |
| Nicotiana benthamina | CNV, RCNMV, and TMV | Mosaic and discoloration on leaves of a wide-range of the host plant | HSP70 HSP90 |
Up | [108,112,113] | |
| Solanum tuberosum | SYNV and INSV | Yellow and black rings, spots and lesions on leaves, and leads to plant death | HSP18 HSP20 |
Up | [114] | |
| Oryza sativa | RSV and RBSDV | Dark green rigid leaves, white- and sometimes black-streaked strips along the leaves, veins and stem | HSP20 HSP70 |
Up | [7,115,116] | |
| Solanum tuberosum | PVY | Potato tuber necrotic rings, spots, disease and decay | HSP | Up | [117] | |
| Bacteria | Nicotiana benthamina | Ralstonia solanacearum | Wide- range of the host, it enters the xylem of a plant and causes wilting | HSP17 | Up | [103] |
| Ralstonia solani | Bacterial wilt by blockade of conducting vessels | HSP90 | Up | [107] | ||
| Citrus spp | Xanthomonas axonopodis pv. citri | This bacterium causes the citrus canker and spots on leaves and blemishes on fruits. | Hsp15.5 | Up | [104] | |
| Capsicum annuum | Xanthomonas campestris pv. Vesicatoria | Causes leaf and fruit spots on peppers and tomatoes. | Hsp16 HSP20 |
Up | ||
| Arabidopsis thaliana | Pseudomonas syringae | Round to irregular brown spots. These spots enlarge and blight the whole leaves. | HSP17 HSP21 HSP23 |
Down | [105,106] | |
| Fungi | Oryza sativa | Magnaporthe grisea | Causes destructive disease of rice, rice blast, rice seedling blight and pitting disease. | HSP16 HSP17.4 HSP18 |
Up | [92] |
| Solanum lycopersicum | Fusarium oxysporum | Wilting, characterized by clearing of veins, marginal necrosis, yellowing of lower leaves, adventitious roots and ultimate death of tomato plants. | HSP20 | Up | [93] | |
| Rhizopus nigricans Ehrenb. | Rhizopus soft rot first appears as water-soaked areas, which then become sunken and gray mold and dusky black spores grow on the fruit surface of tomatoes. | HSP17.6 | Up | [94] | ||
| Oidium neolycopersici | Fungus that causes white powdery lesions and powdery mildew on tomatoes | HSP72 HSP75 |
Up | [99] | ||
| Arabidopsis thaliana | Rhizoctonia solani | Fungus which causes collar rot, root rot and damping off | HSP17.4 HSP17.6 |
Up | [86,87] | |
| Malus domestica | Venturia inaequalis | Fungus causes scab disease on apples and pears | HSP21 | Down | [98] | |
| Solanum tuberosum | Phytophthora infestans | Fungus causes late blight of potatoes | HSP17.8 HSP70 |
Up Up |
[100] [101] |
|
| Hordeum vulgare | Blumeria graminis f. sp. hordei | Fungus causes powdery mildew on grasses and cereals like barley. | Hsp16.9 Hsp17.5. |
Up | [118] | |
| Nematodes | Gossypium hirsutum | Roylenchulus reniformis | Stunted growth, root necrosis and the plant shows symptoms similar to nutrient and water deficiency | 54 HSP | Up | [7] |
| Glycine max | Meloidogyne javanica | Root knot on tropical crops, it is causes irregular galls and swollen roots | HSP22.4 HSP17.9 HSP17.9 HSP22.4 |
Up | [119,120] | |
| Heterodera glycines | Forms cysts on the roots of soybeans. It causes chlorosis of leaves and stem and root necrosis | HSP20 | Up | [121] | ||
| Helianthus annuus | Meloidogyne incognita | Irregular galls on the root of sunflowers | HSP17.6 HSP17.7 HSP18.6 |
Up | [122,123] | |
| Solanum lycopersicum | Meloidogyne spp. | Knots and galls on the roots of tomatoes, swollen roots and dwarf stem | HSP90 | Up | [124] | |
| Nicotiana benthamina | Meloidogyne incognita | Root knot and galls on tobacco roots and causes wilting of leaves | HSP90 | Up | [125] |
Turnip vein clearing virus (TVCTV), Oilseed rape virus (ORTV), Potato virus X (PVX), Cucumber mosaic virus (CMV), Turnip mosaic virus (TuMV), Tomato yellow leaf curl virus (TYLCV), Cucumber Necrosis Virus (CNV), Red clover necrotic mosaic virus (RCNMV), Tobacco mosaic virus (TMV), Sonchus yellow net virus (SYNV), Impatiens necrotic spot virus (INSV), Rice stripe virus (RSV), Rice black Streaked dwarf Virus (RBSDV), Potato virus Y (PVY).
.2. Abiotic Stress Tolerance
Abiotic stresses are extreme environmental conditions like extreme temperatures, water deficit, and ion imbalance due to heavy metals and salinity, which pose a serious threat to plants survival, yield and quality. Global warming and climate change, due to industrialization, has further worsened the situation. Greenhouse gases, particularly the concentration of CO2, is increasing constantly in the atmosphere, which is estimated to reach 520 ppm from 410 by the year 2100 [36]. Twenty per cent of the world cultivated land and almost 50% of irrigated land is affected by salinity, and yield loss up to 50% due to drought is projected by the year 2050 [128]. The world population by the year 2050 is approximated to be 9 billion [129], so, in such a situation, biotic and abiotic stress-tolerant cultivars need to be developed using transgenic and omic techniques. The role of HSPs has been studied by various scientists under different abiotic stresses (Table 3).
Table 3. Summary of studies of plant HSP and abiotic stresses.
| Abiotic Factors | Plant | Type of HSP | Expression Pattern | Technique Used | Reference |
|---|---|---|---|---|---|
| High temperature stress | Wheat | HSP70 | up | qRT-PCR | [158] |
| HSP26 | up | qRT-PCR | [183] | ||
| Rice | HSP100 | up | WB | [134] | |
| HSP90 | up | q-PCR | [145,146,184] | ||
| Maize | HSP101 | up | SDS-PAGE | [63,65] | |
| HSP70, HSP17.6 | up | SDS-PAGE | [139] | ||
| Arabidopsis | HSP101 | up | qRT-PCR | [127,128,129,130] | |
| HSP100 | up | SDS-PAGE | [65] | ||
| HSP90 | up | qRT-PCR, WB | [147,185] | ||
| HSP70 | up | qRT-PCR | [186] | ||
| HSP60 HSP70 |
up | qRT-PCR | [148] | ||
| Potato | HSP70 | up | qRT-PCR | [156] | |
| Tomato | HSP70 | up | SDS-PAGE, WB | [140] | |
| HSP20 | up/down | qRT-PCR | [187] | ||
| Pea | HSP17.9 HSP18.1 |
up | qRT-PCR | [188] | |
| HSP70 | up | qRT-PCR | [154] | ||
| Pepper | HSP70 | up/down | qRT-PCR | [151] | |
| HSP70 | up | qRT-PCR | [153] | ||
| HSP60 | up | qRT-PCR | [189] | ||
| HSP20 | up/down | qRT-PCR | [78] | ||
| HSP16.4 | up | qRT-PCR | [152] | ||
| Soybean | HSP90 | up | qRT-PCR | [146] | |
| Cabbage | HSP70 | up | qRT-PCR | [155] | |
| Tea | All HSPs | up | qRT-PCR | [159] | |
| Witch grass | HSP70 | up | MA, qRT-PCR | [149] | |
| Alfalfa | HSP70 | up | qRT-PCR | [150] | |
| Foxtail millet | HSP20 | down | qRT-PCR | [53] | |
| Chrysanthemum | HSP70 | up | MS, qRT-PCR | [157] | |
| Low temperature stress | Arabidopsis | HSP70 | up | MS, qRT-PCR | [169] |
| Tobacco | HSP70 | up | MS, qRT-PCR | [161,165] | |
| Maize | HSP70 | up | MA, qRT-PCR | [171] | |
| Wheat | HSP70 | up | MS | [190] | |
| HSP90 | up | MS | [175] | ||
| HSP60 HSP21 |
down | MS, qRT-PCR | [167] | ||
| Rice | HSP75 HSP95 |
up | MS, qRT-PCR | [180] | |
| HSP90 | up | MS | [191] | ||
| Barley | HSP70 | up | MS | [167] | |
| Chicory | All HSPs | up | MS | [173] | |
| Rape seed | HSP90 | up | qRT-PCR | [172] | |
| Poplar | HSP70 HSP90 |
up | MS | [178] | |
| Pea | HSP70 | up | MS | [177] | |
| Sunflower | HSP60 HSP21 |
down | MS, qRT-PCR | [181] | |
| Tomato | HSP110 HSP70 |
up | qRT-PCR | [192] | |
| Plum | HSP20 | up | qRT-PCR | [193] | |
| Grape | HSP18 HSP22 |
up | qRT-PCR | [194] | |
| Salinity stress | Wheat | HSP70 | up | MS | [195] |
| All HSPs | up | [196] | |||
| Rice | HSP70 | up | qRT-PCR | [197,198] | |
| HSP40 | up | qRT-PCR | [165] | ||
| ClpD1 | up | qRT-PCR | [199] | ||
| Arabidopsis | HSP90.2 HSP90.5 HSP90.7 |
up | qRT-PCR | [200] [146] |
|
| Rose | 17.8 | up | qRT-PCR | [16] | |
| Soybean | HSP90 HSP70 HSP60 HSP20 |
up/down | MS | [201] [202] |
|
| Poplar | HSP100 HSP90 HSP70 HSP60 HSP40 HSP20 |
up | qRT-PCR | [203] | |
| Drought stress | Arabidopsis | HSP70 | up | qRT-PCR | [204] |
| Tobacco | HSP70 BiPs |
up | qRT-PCR | [205] [176] |
|
| Barley | HSP17.5 | up | qRT-PCR | [206] | |
| Rice | HSP70 | up | MA, MS, qRT-PCR | [207] | |
| HSP101 | up | MS | [208] | ||
| HSP17.7 | up | qRT-PCR | [209] | ||
| Maize | HSP70 HSP26 |
up | MS | [39] | |
| Pepper | HSP16.4 | up | qRT-PCR | [152] | |
| Chickpea | HSP70 | up | MS, qRT-PCR | [210] | |
| Sugarcane | HSP70 | up | qRT-PCR | [211] | |
| Cotton | All HSPs | up | qRT-PCR, WB | [212] | |
| Kentucky grass | HSP70 | up | MS | [160] | |
| Poplar | HSP70 | up | MS | [213] | |
| Light stress | Arabidopsis | HSP70 | up | qRT-PCR | [214,215] |
| Goose foot plant | HSP23 | up | SDS-PAGE | [69] | |
| Chlamydomonas | HSP70 | up | MA | [216] | |
| Heavy metal stress | Tomato | HSP70 | up | MS, SDS-PAGE | [217] |
| Rice | HSP70 BiPs |
up | MS, SDS-PAGE | [218] | |
| HSP80 HSP17.9 |
up | MA | [219] | ||
| Poplar | HSP70 | up | qRT-PCR | [212] | |
| Soybean | HSP26 | up | qRT-PCR | [220] | |
| Carrot | HSP17.7 | up | qRT-PCR | [221] | |
| Flaxseed | HSP70 | up | MA, MS | [168] | |
| HSP80 | down | ||||
| Arabidopsis | HSP70 | up | qRT-PCR, NB, MS | [222] | |
| Bird foot trefoil | HSP90 | up | qRT-PCR | [223] | |
| Flooding stress | Arabidopsis | HSP101 HSP70 |
up | qRT-PCR | [224] |
| Tomato | HSP23.6 | up | qRT-PCR | [225] | |
| Rice | HSP70 | up | qRT-PCR | [226] | |
| Maize | HSP70 | up | qRT-PCR, WB | [227] | |
| Soybean | HSP70 | up | qRT-PCR, SDS-PAGE | [228] | |
| HSP60 | up | MS | [229] |
Quantitative real-time polymerase chain reaction (qRT-PCR), Mass spectrometry (MS), Microarray (MA), Western blotting (WB), Northern blotting (NB), Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
This entry is adapted from the peer-reviewed paper 10.3390/ijms20215321
This entry is offline, you can click here to edit this entry!
