The observation that an application of a pulsed electric field (PEF) resulted in an increased permeability of the cell membrane has led to the discovery of the phenomenon called electroporation (EP). Depending on the parameters of the electric current and cell features, electroporation can be either reversible or irreversible. The irreversible electroporation (IRE) found its use in urology as a non-thermal ablative method of prostate and renal cancer. As its mechanism is based on the permeabilization of cell membrane phospholipids, IRE (as well as other treatments based on EP) provides selectivity sparing extracellular proteins and matrix. Reversible EP enables the transfer of genes, drugs, and small exogenous proteins. In clinical practice, reversible EP can locally increase the uptake of cytotoxic drugs such as cisplatin and bleomycin. This approach is known as electrochemotherapy (ECT). Few in vivo and in vitro trials of ECT have been performed on urological cancers. EP provides the possibility of transmission of genes across the cell membrane. As the protocols of gene electrotransfer (GET) over the last few years have improved, EP has become a well-known technique for non-viral cell transfection. GET involves DNA transfection directly to the cancer or the host skin and muscle tissue. Among urological cancers, the GET of several plasmids encoding prostate cancer antigens has been investigated in clinical trials.
- electroporation
- therapy
- cancer
- urology
- electrochemotherapy
- drug delivery
- Introduction
The prevalence of kidney, prostate, and bladder cancer is increasing rapidly within the context of an ageing population [1]. Urological cancers are generally regarded as a problem predominantly concerning wealthier countries. However, risk factors such as tobacco smoking, diet, and lifestyle inevitably lead to increased prevalence among lower-income populations [1]. Easily available, safe, and economical therapies must be developed to reduce the degree of inequality in terms of incident cases and deaths due to urological cancers. The technique known as electroporation provides the possibility for efficient focal ablation, intracellular delivery of cytostatics or other molecules such as calcium, and safe gene transfection. In its simplicity, no sophisticated equipment is required, except a pulse generator and an electrode.
The present development of sensitive methods of imaging, such as multiparametric magnetic resonance imaging (mpMRI), enables precise tumor localization, biopsy guidance, and focal therapy [2]. It is estimated that one-third of patients with mpMRI-detected and biopsy-proven lesions in the prostate gland are potential candidates for focal treatment [3]. For this group of patients, methods based on electroporation, irreversible electroporation (IRE), or electrochemotherapy (ECT) constitute possible choices of treatment.
With the increasing importance of immune therapy, new gene delivery techniques are being developed at a very fast pace. Among viral and non-viral approaches, gene electrotransfer (GET) is characterized by a high safety profile, acceptable efficiency, availability, and ease of application [4]. In vivo trials of anti-tumor therapy for urological cancers have already proven their efficacy
There are few publications dedicated to physicians describing the more detailed mechanism of electroporation and the possibilities of its applications in urology. It should not be neglected that a few vulnerabilities of electroporation (EP) still require new solutions and need to be optimized. The underlying mechanisms should be discussed more frequently to increase awareness of physicians applying this method in the clinic and to illustrate its potential for future development.
- The Theoretical Background of EP
Physiologically all human cells possess a resting voltage on their plasma membrane ranging from −70 mV to −30 mV, which is generally provided by Na+ and K+ active and passive transport through the membrane [5]. The foundation of the EP was the observation that cells exposed to an external electric field change the properties of their membranes and become more permeable [6,7]. Once the cell is exposed to an external electric field, an additional component of the voltage across the membrane occurs [8]. The induced transmembrane voltage for a spherical cell with a nonconductive membrane can be calculated with Laplace’s equation (1):
|
∆Φm = 3/2ERcosθ |
(1) |
where E is the electric field in the region of a cell, R is cell radius, and θ is the angle measured from the center of the cell to the direction of the field [8].
When the transmembrane potential reaches 200–250 mV, parts of the membrane become highly permeable [9,10]. Permeabilization is a local process, and the fraction of the permeabilized membrane strongly correlates with the electric field intensity [11,12]. The organization of the membranes permeable spots is inhomogeneous [12].
To permeabilize the cell membrane, usually, a series of electrical rectangular pulses are applied [13]. Depending on the pulse duration, there could be distinguished nanosecond, microsecond, or millisecond electroporation protocols [14]. Today, microsecond pulses find clinical application in IRE for the ablation therapy of prostate cancer [15]. The use of microsecond electric pulse in IRE for other urological tumors, such as kidney cancer (NCT01967407, NCT02828709, NCT02298608) and urinary bladder neoplasm (NCT02430623), is continually being tested in clinical trials. Further considering urological tumors, nanosecond electric pulses are studied only at the in vitro level [16].
The precise molecular mechanism of EP is not fully understood. However, some properties of pulsed electric fields (PEFs), as well as cell features affecting the electropermeabilization, remain defined. The influence of the external electric field on the transmembrane voltage varies depending on the shape of the cells. The position and orientation of cells to the electric field also determines the transmembrane voltage [17]. The density of defects in the cell membrane depend on pulse duration and number of pulses [12,13,18].
The following three modalities of electric-field-aided treatment methods can be distinguished: irreversible electroporation, electrochemotherapy and gene electrotransfer. Each of them is based on the use of a pulsed electric field which induces changes in the membranes of the target cells, making them more permeable. However, each technique differs in mechanism of action, uses different parameters of the electric field, and has varied aims of application in the clinical setting. This will be continually expanded upon and described in the following sections.
The process of electroporation is complex and should not be simplified only to the phenomenon of permeabilizing the cell membrane. The electrical field affect cell permeability and also generates a transient mechanical force that stretches the spherical membrane [19]. EP may lead to cytoskeleton destabilization and changes in membrane elasticity [20]. It was observed that electrical pulses generate reactive oxygen species at the permeabilized loci [21]. However, Michel et al. proved that even if reactive oxygen species appear, their presence does not induce membrane permeabilization [22]. Electropermeabilization is followed by water flux and, consequently, by the osmotic swelling of cells [23]. When the cellular membrane becomes permeable, the leakage of metabolites such as ATP from the cytoplasm can be observed [24]. The intracellular ATP content is strongly related to the viability of the cells after electropermeabilization [25].
After permeabilization, the transfer of molecules such as drugs or even the insertion of exogenous proteins into the cell interior is possible [26,27]. The transfer of small molecules across the permeabilized membrane is driven by two factors—mainly by the concentration gradient across the membrane, but also by the post-pulse transmembrane potential [28]. EP enables the molecules to flow for seconds and up to a few minutes after the pulse [18]. The persistence of cells permeable state depends on the pulse duration and the number of pulses applied [29]. When applied PEFs do not overcome the irreversible electroporation threshold, the resealing of the cell membrane occurs [30–32]. The process engages multiple mechanisms. It was proven that cellular proteins and parallel the processes of endo and exocytosis contribute to cell membrane repair [33].
Generally, electroporation of tissue due to their inhomogeneity and anisotropy is difficult to foresee [34]. In electroporation, tissues should be considered as an insulator and conductor [35]. A few basic physical terms need to be reminded to clarify the dielectric properties of tissues. Permittivity is the measure of the capacitance of the material to store an electric field in the polarization of the medium. Conductivity is a measure of the ability of the material to conduct an electric current. Impedance is a measure of the opposition to the electric current in an electric circuit.
Conductivity and permittivity of tissues depend on the frequency of the applied electric pulses. Therefore, the tissue permittivity decreases in higher frequencies, and the conductivity increases. As the cell membrane becomes permeabilized, it increases its conductance [36]. The local changes in the electric field should be taken into account during individualized, patient-specific treatment planning [37]. It was observed that tumors are characterized by higher conductivity in comparison to normal tissues, probably due to regions of necrosis [38]. The phenomenon that the conductivity increases with electroporation enables it to effectively electroporate deeper structures of the tissue using lower voltages [39]. By measuring the electric conductivity changes in tissues, the cell permeabilization threshold can be estimated [40].
Controlling the real-time changes of the tissue impedance by the use of electrical impedance tomography (EIT) enables users to estimate the electroporated area [41]. Electrodes detect the changes in impedance caused by electropermeabilization. Subsequently, the obtained data is being transformed into the image of the electroporated area. Another technology—magnetic resonance electrical impedance tomography (MREIT)—combines EIT and magnetic resonance imaging (MRI). MREIT algorithms transfer the MRI image to digitally reconstruct the conductivity distribution inside the tissue. In contrast to EIT, it avoids the implementation of additional electrodes. In vivo and ex vivo research shows that MREIT can be applied in clinical electroporation-based procedures to improve the security of therapies [42,43]. In the future, we can expect the introduction of this technology in clinical settings.
As tissues are diverse and anisotropic, they possess distinct conduction properties [44]. Various tissues are characterized by a different proportion of the extracellular matrix, different water content, and irregular vascularization [35]. Inhomogeneity of conduction depends on the placement of the electrodes with respect to the major axis of tissue. If the tissue is organized in fibers, the detectable discrepancy of the longitudinal and transverse conductivity may occur [34]. In the longitudinal orientation of electrodes, the electricity is directed by cells organized in fibers, whereas in the transverse orientation, the charge has to overcome an extracellular matrix, which is less conductive than cells [35].
The pre-treatment computer simulation is helpful to plan the therapy, optimize pulse parameters, choose appropriate electrodes, and determinate their placement inside the tissue. Numerical modeling can be applied to predict the electric field distribution in inhomogeneous biological tissues. The simulation enables clinicians to visualize the electric field density in the targeted region and to determinate the range of irreversible and reversible electroporation and to determine the temperature rise occurring due to Joule heating [45].
- IRE
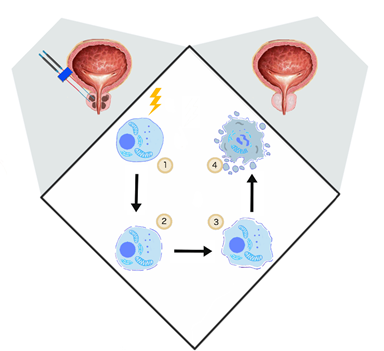
Davalos et al. showed that the application of PEFs promoting irreversible defects in the cellular lipid bilayer could be applied as a novel ablation method [46]. In contrast to other thermal tumor ablation possibilities such as microwave ablation, high-intensity focused ultrasound, or cryoablation, IRE is based on electropermeabilization and thus causes no excessive thermal effect [47]. (Figure 1)
Figure 1. The general mechanism of irreversible electroporation (IRE). (1) Delivery of short electrical impulses (10–90 impulses 1000–2500 V/cm, 50–100 μs) [48], (2) cancer cell permeabilization, (3) irreversible permeabilization results in osmotic swelling [23], cytoskeleton destabilization [20], ATP depletion [24], (4) cell turns necrotic [46].
If the electric field strength is too far above the permeabilization threshold value, the permeabilized state is irreversible and results in cell destruction [46]. IRE does not require the application of chemotherapeutic agents. Several experiments investigated the specificity of IRE. It was shown that even if IRE has the potential to affect the nerves, the axonal regeneration process occurred two months after the procedure [49]. Another study on prostate gland ablation confirmed the preservation of the urethral wall, nerves, and vessels [15]. With optimal electric field parameters, IRE predominantly affects cell membrane phospholipids. Extracellular proteins, and the cell matrix are usually not affected [50].
Usually, the transmembrane potential of 1 V is sufficient to generate irreversible electroporation [46]. IRE is considered to be a non-thermal ablative method. However, the thermal effect occurs due to Joule heating and cannot be neglected. The amount of heat released is proportional to the electrode spacing and diameter and depends on repetition frequency [51]. Electrode configuration, the distance between electrodes, and the active tip length are the other factors that influence the IRE ablation zone. However, those features can be modulated during the procedure [52]. Due to the complexity of in vivo IRE procedure, pulse parameters have been established mainly experimentally. In in vivo studies, the electric field between 1000 V/cm and 2500 V/cm has been applied for IRE of the tumor. In most of the trials, the pulse durations ranged from 50 μs to 100 μs, and the pulse number varied between 10 and 90 [48].
Although IRE presents many advantages compared to other focal thermal ablation methods, there are a few issues that limit it in its clinical use. The successive ablation requires precise and parallel placement of multiple electrodes to optimize the ablation zone. IRE parameters should be personalized, since when adequately adjusted, can limit the damage caused by heat and extend the tissue ablation zone [46]. IRE is known to be minimally invasive. Nevertheless, it still requires general anesthesia and complete muscle relaxation [53]. Muscle contraction during the delivery of impulses can displace electrodes [54]. Moreover, to avoid cardiac arrhythmias, the electrical pulses need to be introduced during the refractory phase, and as a consequence, the electrode device should be synchronized with electrocardiography (ECG) [55].
In clinical practice, four different types of electrodes are used for IRE: needle, catheter, plate, and clamp. They can be applied percutaneously or intraoperatively. The endoscopic approaches for IRE ablations are currently still under development [56]. Furthermore, the endovascular IRE has been investigated for vascular smooth muscle cells. This minimally invasive method is used for creating a suitable niche for exogenous cell engraftment in regenerative surgery [57,58].
CT, MRI, and ultrasounds are often used for electroporation imaging. However, those methods cannot precisely estimate the electroporated area during the delivery of impulses [59–61]. The real-time imaging can be achieved by MREIT, which is a novel method was shortly described above.
- Electrochemotherapy
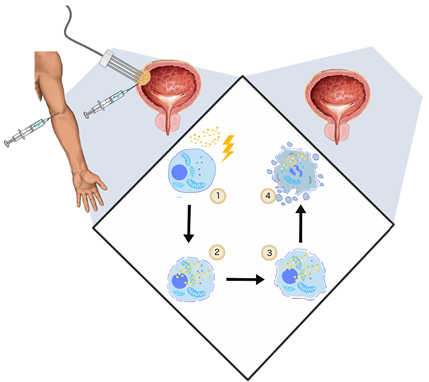
The application of the electric fields to enhance the intracellular anti-cancer drug uptake was studied and described by Mir et al. in 1991 [104]. Uptake of membrane non-permeable drugs can be locally increased when their intravenous or intratumoral administration is followed by electroporation. Clinically, in an approach commonly called electrochemotherapy, cisplatin and bleomycin are used [105]. Apart from cytostatics, intracellular uptake of calcium ions can be enhanced with electroporation as well. This novel solution showed no systemic toxicity and high efficiency in the in vitro and in vivo treatment of various cancer types [106]. In standard ECT, trains of eight 100-μs-long pulses are applied to achieve the reversible permeabilization of cells. The electroporation-mediated internalization of chemotherapeutics involves different mechanisms. During the application of PEFs, charged molecules cross the plasma membrane moved by electrophoretic forces. Subsequently, once the cell is in the permeable state, small hydrophilic molecules can enter the cell interior diffusing through the permeabilized area [107]. ECT anti-tumor effect cannot be explained only by increased uptake of cytostatics. Firstly, ECT showed high efficiency in in vivo studies as it stimulates the immune response. After the immunogenic death of electroporated cells, cancer antigens can be captured and recognized by dendritic cells and eventually increase antitumor response [108]. ECT could be considered as the adjuvant immunogen electrotransfer to peritumoral tissue [109]. The process leads to the local effects and triggers the systematic response against the metastases–abscopal effect [110]. Second, electroporation causes an increase in vessel permeability and constriction of arterioles resulting in so-called “vascular lock” [32]. The effect provides the targeted accumulation of the intravenously administrated drug [111] (Figure 2).
Figure 2. The general mechanism of ECT. (1) Two steps of ECT—administration of the drug (cisplatin, bleomycin) and application of PEFs (1000 V/cm, 100 μs, 9); (2) once the cell membrane gets permeabilized, the cytostatic drug penetrates to cell interior; (3) initiation of cell death by drug cytotoxicity, systemic anti-tumor reaction [108]; vasoconstriction, thus the ischemia of cancer cells [112] (4); and cell turns necrotic/apoptotic.
The data of ECT on urological cancers still remain restricted. However, with promising in vivo results, together with the technical progress of endoscopic or percutaneous approaches, the introduction of ECT in urology in the nearest future should to be expected.
This entry is adapted from the peer-reviewed paper 10.3390/cancers12082208