Tricuspid atresia (TA) is a cyanotic, congenital heart defect (CHD) and is defined as congenital absence or agenesis of the morphologic tricuspid valve. It is the third most common cyanotic CHD, and is the most common cause of cyanosis with left ventricular hypertrophy. The prevalence of TA is estimated to be 2.9% of autopsy cases and 1.4% to 1.5% of the clinical cases of CHD.
- echocardiography
- Doppler
- tricuspid atresia
- Fontan
- Bidirectional Glenn
- Modified BT shunt
1. Anatomic Features of TA
TA has been classified on the basis of valve morphology ( Figure 1 ) [1][2][3], pulmonary vascular markings on chest roentgenogram [4], and associated cardiac defects ( Figure 2 ) [1][5][6][7][8]. On the basis of the morphologic appearance of the atretic tricuspid valve, it has been characterized as muscular, membranous, valvular, Ebstein’s, unguarded with muscular shelf, and atrioventricular canal types ( Figure 1 ) [1][3][9]. The muscular type is the most common variety and constitutes 89% of all TA cases [1][9][10]; other types occur with much lower frequency, as shown in Figure 1 . The classification based on associated cardiac defects largely centers on the interrelationship of the great arteries ( Figure 2 , top): Type I, normally related great arteries; Type II, d-transposition of the great arteries; Type III, malpositions of the great arteries other than d-transposition; and Type IV, truncus arteriosus. Each type is again divided into subgroups on the basis of the pulmonary artery anatomy ( Figure 2 , bottom): subgroups—a. pulmonary atresia, b. pulmonary stenosis or hypoplasia, and c. normal pulmonary arteries (no pulmonary stenosis). It is generally thought that classification based on associated cardiac defects is more useful clinically [1][10].
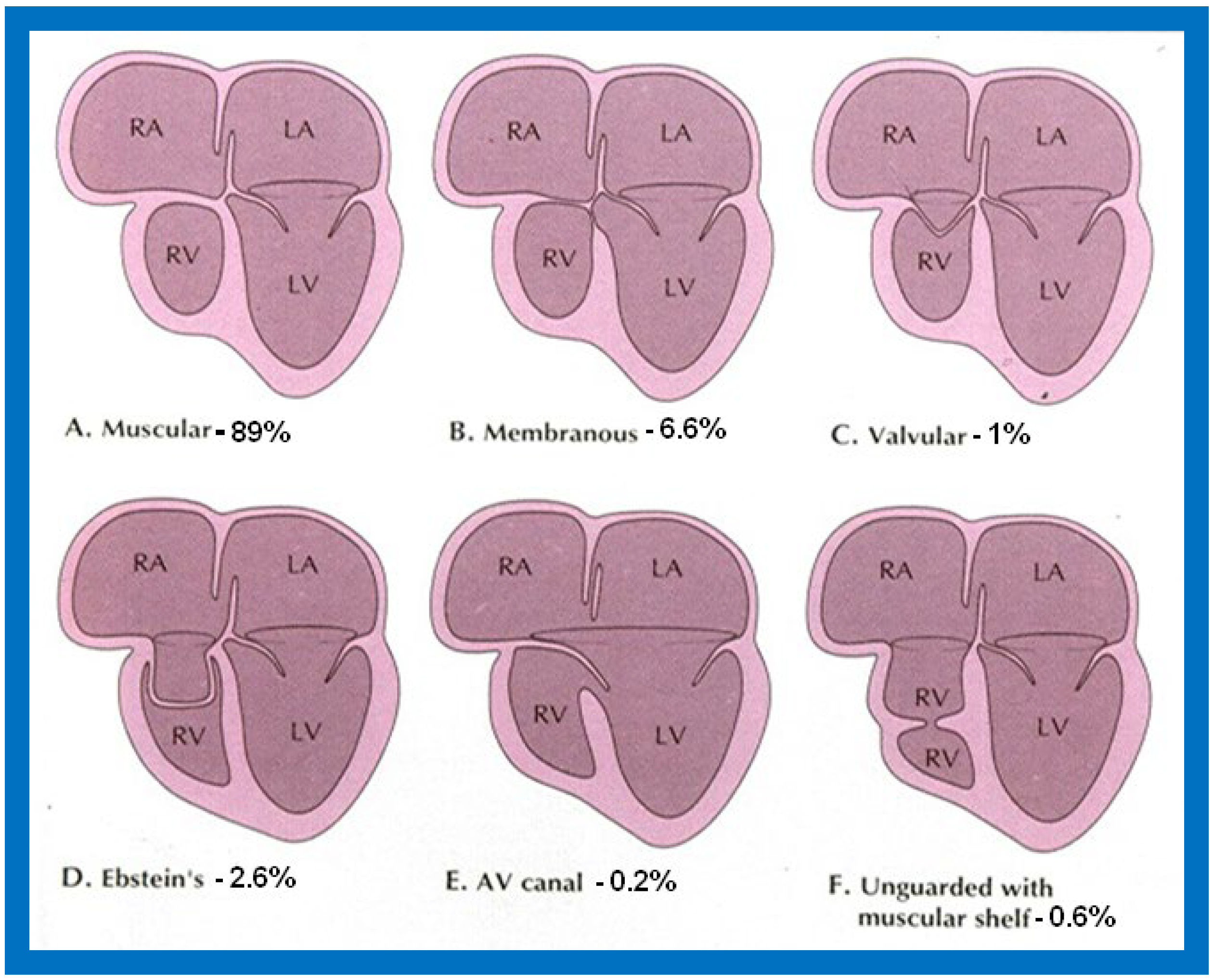
Figure 1. Diagrammatic portrayal of anatomic types of tricuspid atresia based on the morphology of the atretic tricuspid valve. (A) muscular type, (B) membranous type, (C) valvular type, (D) Ebstein’s type, (E) atrioventricular canal type, and (F) unguarded valve with muscular shelf. The prevalence of each type is shown under each diagram. For the sake of simplicity, the great vessels are not shown. Also note that no ventricular septal defects are shown. LA, left atrium; LV, left ventricle; RA, right atrium; RV right ventricle. Modified from Rao PS, Alapati S. Neonatology Today 2012;7(5):1–12 [8].
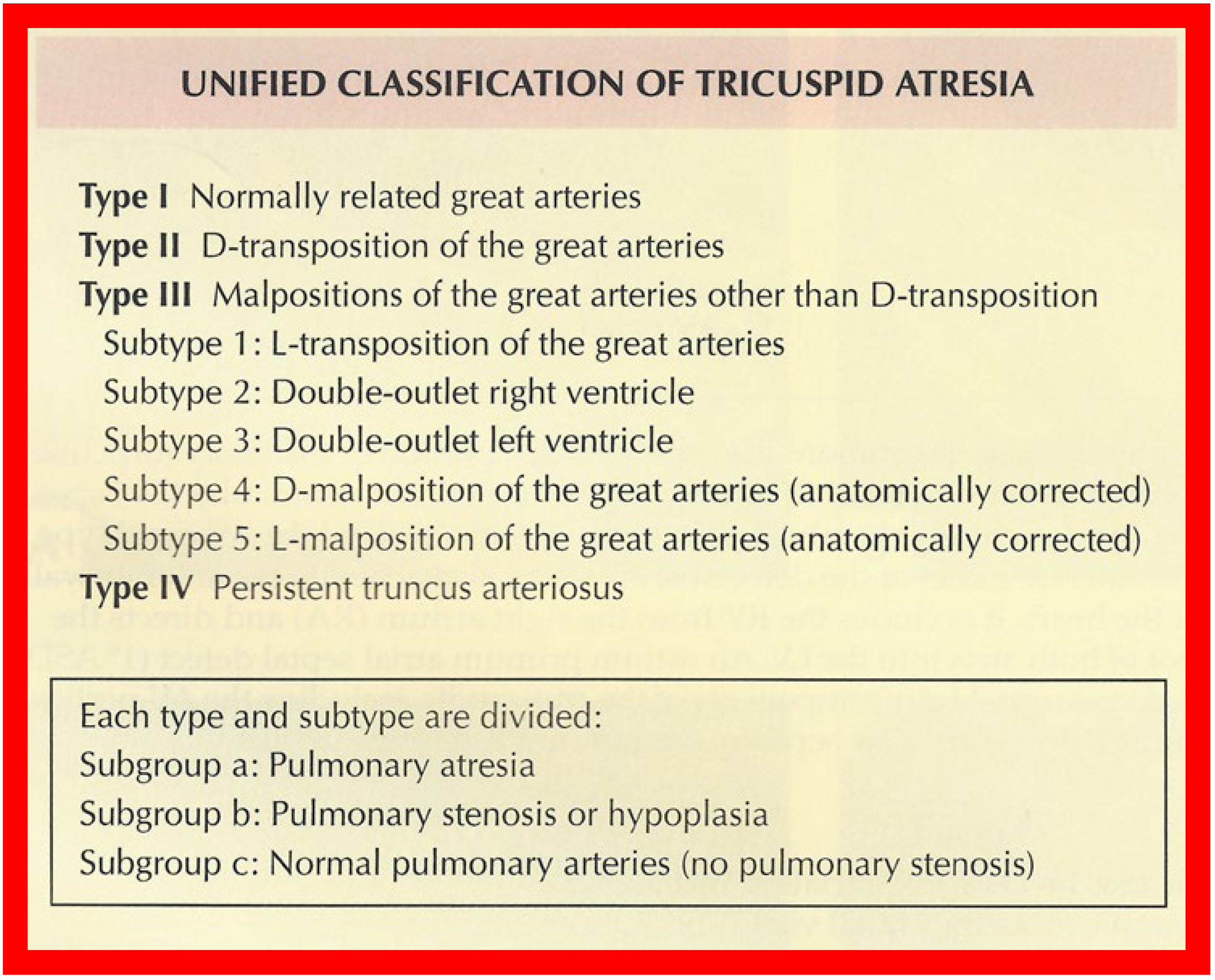
Figure 2. Classification of Tricuspid Atresia. Reproduced from [9].
The right atrium (RA) is enlarged and the atretic tricuspid valve is seen as a dimple or a localized fibrous thickening in the floor of the right atrium at the anticipated site of the tricuspid valve [7]. An inter-atrial defect is necessary for survival and is typically a stretched patent foramen ovale (PFO). However, occasionally, an ostium secundum atrial septal defect (ASD) or an ostium primum ASD may be present, facilitating right to left shunt. The left atrium (LA) is enlarged, especially when the pulmonary blood flow is increased. The mitral valve is usually bicuspid and, morphologically, a mitral valve. However, its orifice is large and occasionally incompetent. The left ventricle (LV) is evidently a morphological LV, but it is enlarged and hypertrophied. The right ventricle (RV) is hypoplastic and is not sufficiently large in size to support pulmonary circulation in most patients.
The ventricular septum is intact in a rare patient, but, usually, a ventricular septal defect (VSD) is present; this may be large or small, or multiple VSDs may be seen. The VSD may be: conoventricular or perimembranous, located inferior to the septal band; a conal septal malalignment type, located in between the anterosuperior and posteroinferior limbs of the septal band; muscular, located inferiorly in the muscular septum; or, rarely, of an atrioventricular canal type. In the author’s personal experience, VSDs are most commonly seen in the muscular septum, are restrictive [11][12][13] and cause subpulmonary obstruction in Type I patients and potential subaortic stenosis in Type II patients [11][12][13][14][15][16][17][18].
The great vessel relationship is variable, as discussed above ( Figure 2 , top). The ascending aorta is either normal or slightly larger than normal. The RV outflow tract is atretic, narrowed, or normal, depending upon the subgroup (a, b, or c). In patients with normally related great arteries ( Figure 2 ), the obstruction to pulmonary blood flow is frequently at the VSD level although subvalvar or valvar (very rare) pulmonary stenosis or the narrow tract of the hypoplastic RV may occasionally be responsible for such obstruction. In cases with the transposition of the great arteries (Type II), the pulmonary obstruction is usually subvalvar. In cases with pulmonary atresia, either a patent ductus arteriosus (PDA) or aortopulmonary collateral vessels are present. Other abnormalities may be present in nearly 30% of tricuspid atresia cases [19]; significant of these are the coarctation of the aorta (in Type II - transposition cases) and persistent left superior vena cava, which have therapeutic implications.
2. Echo-Doppler Features of TA on the Basis of Current Technology
On 2D echocardiography, the atretic tricuspid valve is visualized directly as a dense band of echoes at the site where the tricuspid valve should be; this echo appearance is that of the most frequent muscular type of TA. This anatomy is better demonstrated in apical and subcostal four chamber views than in other views. The other anatomic types ( Figure 1 ), namely, membranous, valvular, Ebstein’s, atrioventricular septal defect, and unguarded tricuspid valve with muscular shelf, are rare and may also be recognized on 2D echocardiography. An example of an atrioventricular septal defect type of TA [20] is demonstrated in Figure 3 ; in this example, a 2D echocardiogram demonstrated an ostium primum ASD with a common atrioventricular valve and a small RV ( Figure 3 a,b); the entry into the RV appeared to be occluded by a leaflet of the common atrioventricular valve. Left ventricular and right atrial cineangiograms confirmed these findings [20]. Evaluation of the crux cordis ( Figure 4 ) on a 2D echocardiogram (subcostal four chamber view) may help to distinguish the various anatomic types ( Figure 1 ) from each other. In the muscular type of tricuspid atresia, a dense band of echoes is seen where the normal tricuspid valve should be ( Figure 4 A). In membranous types of tricuspid atresia, a thin membrane is seen instead ( Figure 4 B). In both these types, the anterior leaflet of the detectable atrioventricular valve is attached to the left side of the interatrial septum ( Figure 4 A,B). In the atrioventricular septal defect type of tricuspid atresia, the crux cordis is abnormal and cannot be identified; the anterior leaflet of the detectable atrioventricular defect is attached to the anterior wall of the heart, and a large atrioventricular valve leaflet occludes the entry of the RA into the RV ( Figure 4 C). Based on these observations, it was concluded that 2D echocardiographic (and angiographic) features help to differentiate the atrioventricular canal type of tricuspid atresia from the classic muscular tricuspid atresia cases [20].
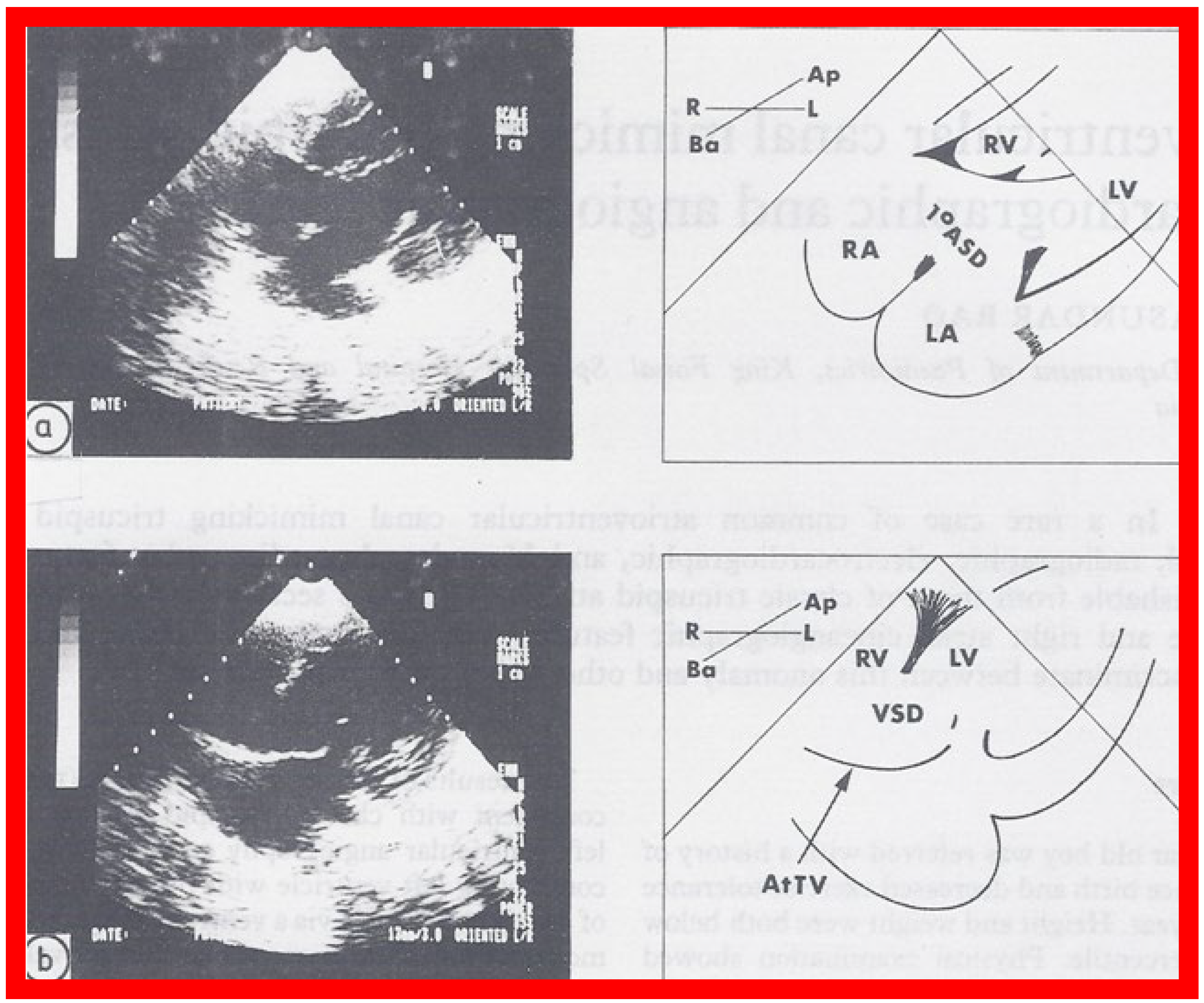
Figure 3. Selected two dimensional, subcostal, four chamber echocardiographic frames with an open (a) and closed (b) atrioventricular valve. Line drawings on the right of (a,b) are made for greater clarity and for labeling. A large ostium primum atrial septal defect (10 ASD) is shown in (a). When the large atrioventricular valve leaflet is open (a), it completely closes the right ventricle (RV) from the right atrium (RA) and the ventricular septal defect (VSD) and allows the emptying of blood from both atria into the left ventricle (LV). When the atrioventricular valve leaflet is closed, (b), it continues to occlude the RV from the RA while allowing the VSD to freely communicate between the RV and LV. Ap, Apex; AtTV, atretic tricuspid valve; Ba, base; L, left; LA, left atrium; R, right. Reproduced from Rao PS. Brit Heart J 1987;58:409–412 [20].
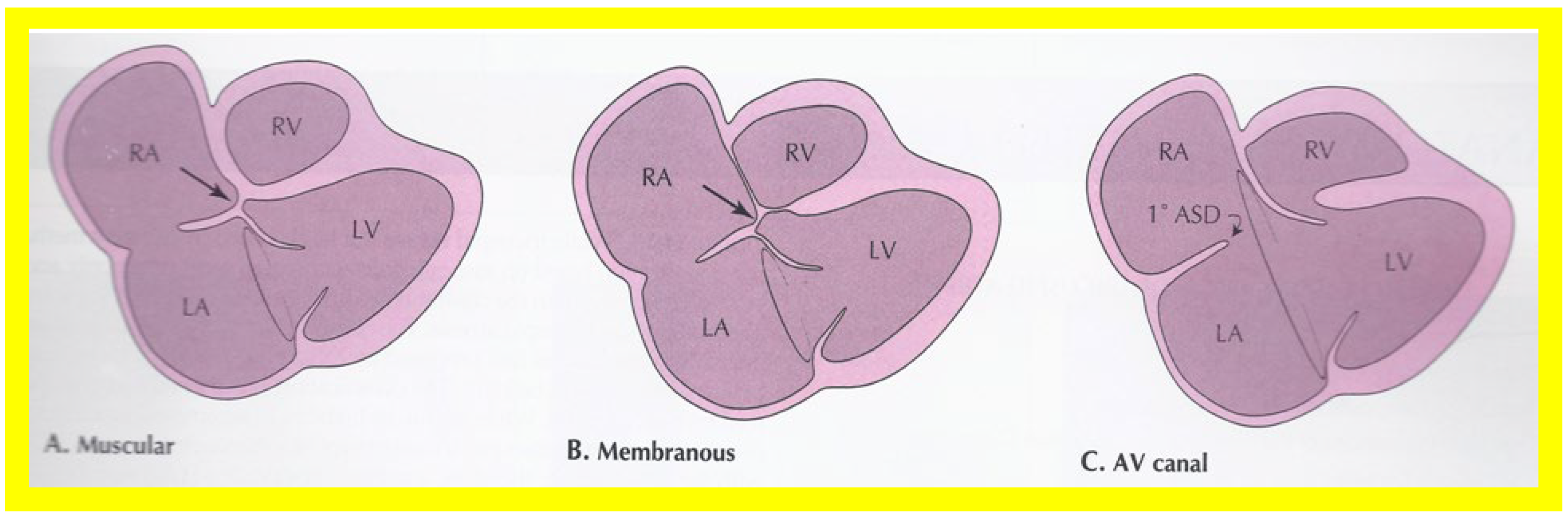
Figure 4. Line drawings demonstrating two dimensional echocardiographic appearances in a subcostal four chamber view of the muscular (A), membranous (B), and atrioventricular canal (C) variants of tricuspid atresia. (A) The atretic tricuspid valve is represented by a thick band of echoes between the right atrium (RA) and the small right ventricle (RV) in the muscular type. (B) The tricuspid valve is represented by a thin line in the membranous type. Note that crux of the heart (arrows in (A,B)) is clearly seen in both these types (A,B). The attachment of the anterior leaflet of the detectable atrioventricular valve to the left side of the interatrial septum is evident. (C) In the atrioventricular canal type of tricuspid atresia, the anterior leaflet of the detectable atrioventricular canal is attached to the anterior wall of the heart, occluding the right ventricle from the right atrium, and allows the exit of blood from both atria into the left ventricle (LV). The crux cordis and the atrioventricular portion of the interventricular septum are not seen. Reproduced from Rao PS. Brit Heart J 1987;58:409–412 [20] and from Reference [9].
Following the demonstration of the atretic tricuspid valve, the sizes of the cardiac chambers are evaluated both by M-mode (Z scores) and 2D echocardiography; an enlarged RA, LA and LV and a small RV are seen. Pulsed (not shown) and color Doppler ( Figure 5 ) studies are helpful in illustrating right to left shunt across a PFO or an ASD.
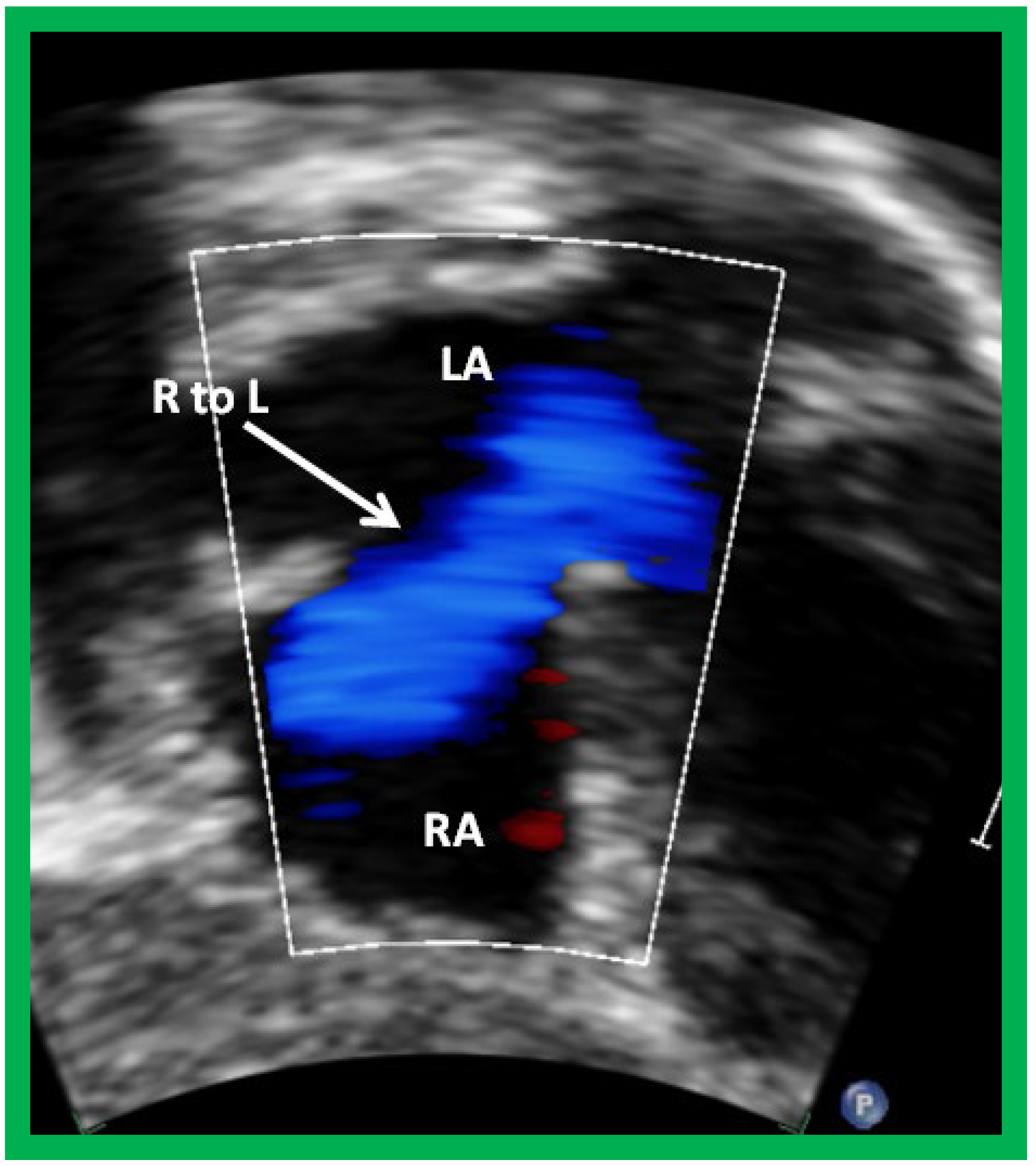
Figure 4. Selected video frame from subcostal view of a patient with tricuspid atresia demonstrating right to left (R to L) shunt (arrow) across the interatrial communication. LA, left atrium; RA, right atrium. Reproduced from Reference [21].
The relationship of the great arteries is examined next in order to classify them into various types, as mentioned above. The relationship of the great arteries (Figure 2, top) is established by following the vessels arising from the ventricles until the pulmonary artery (PA) bifurcation or aortic arch. In Type I patients with normally related great arteries, the aorta arises from the LV (Figure 5) and in Type II patients with transposition of the great arteries, the PA arises from the LV (Figure 6; Figure 7). In Type II patients, the blood vessel arising from the LV should be traced to demonstrate its branching into the right and left PAs (Figure 6; Figure 7). In Type III patients, it may be a little more difficult to assign the great artery relationship and, sometimes, other imaging studies, including angiography, may be needed to define the great artery relationship. In Type IV with truncus arteriosus, the limited data suggest that this can be performed by echocardiography (Figure 8; Figure 15). In the example shown [22], the atretic tricuspid valve (Figure 8a and Figure 9a), VSD (Figure 8b and Figure 9b), hypoplastic RV (Figure 9a), single vessel (truncus) arising from the heart (Figure 8c,d, and Figure 9c,d), and origin of the PA and its division into branch PAs (Figure 8d, and Figure 9c,d) were demonstrated.
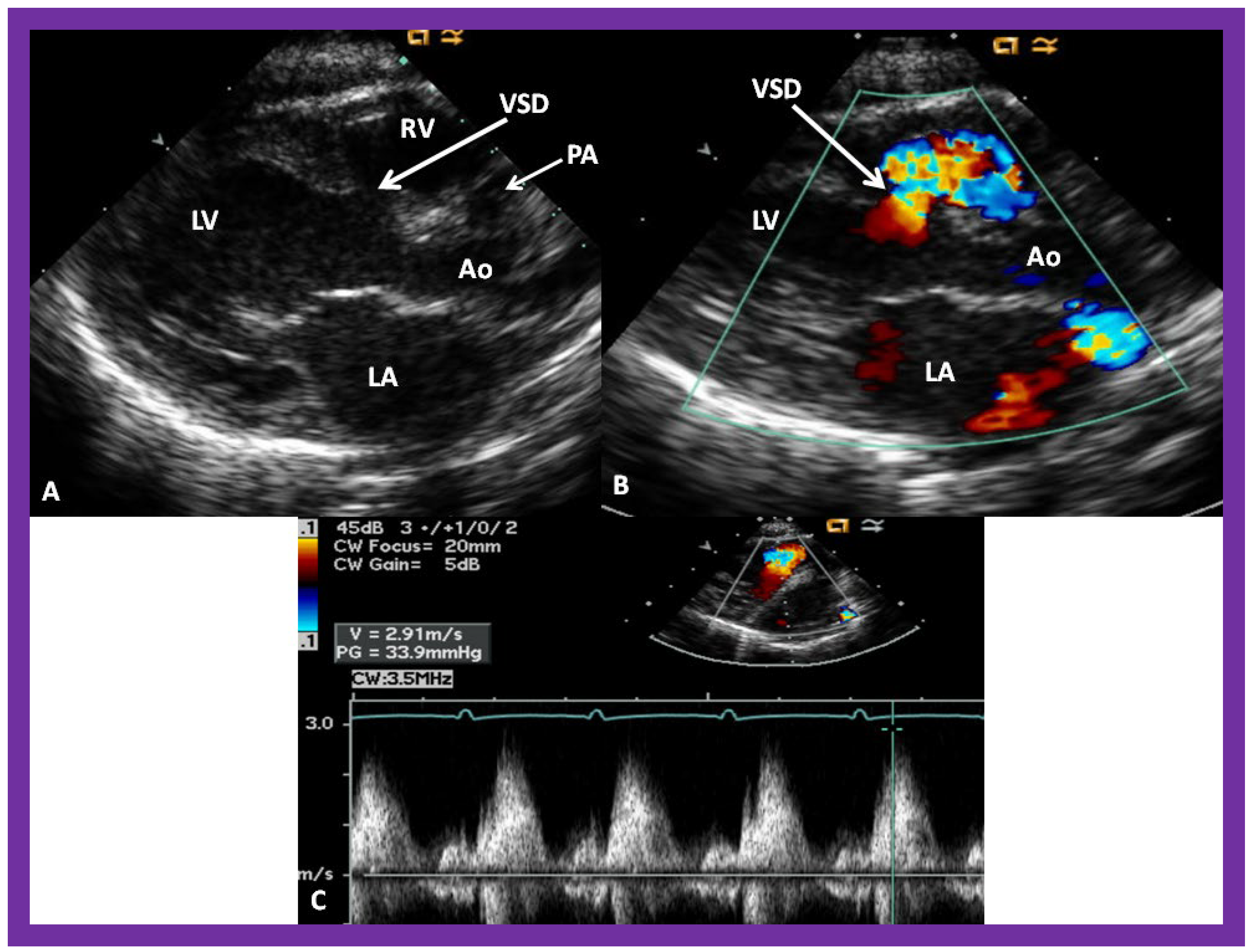
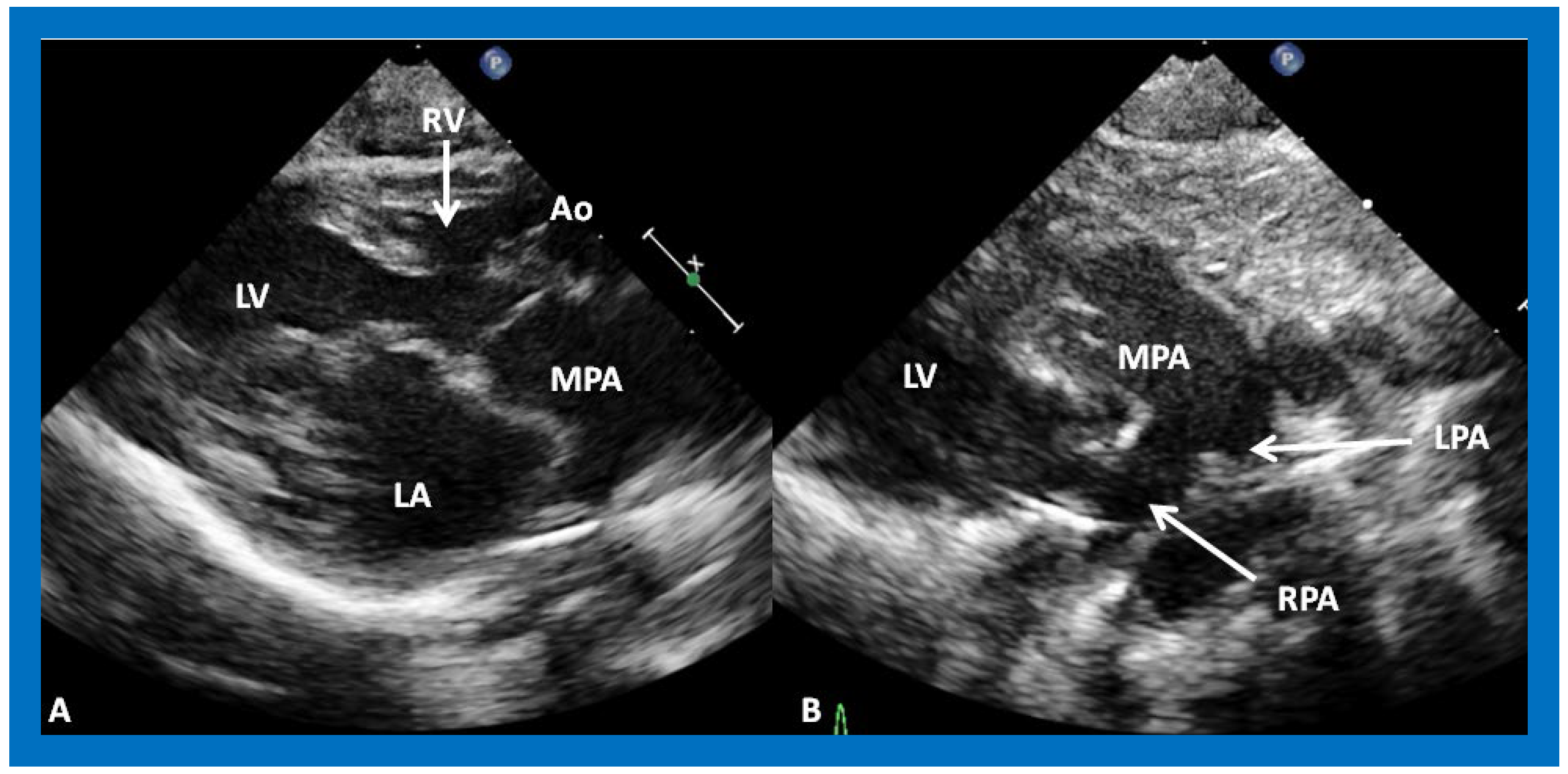
Figure 6. (A) A selected video frame from parasternal long axis views of a patient with tricuspid atresia and transposition of the great arteries demonstrating the left atrium (LA), left ventricle (LV), a very small right ventricle (RV) and a moderate sized ventricular septal defect (not marked). The vessel coming off the LV is traced in (B) and shown to bifurcate into the left (LPA) and right (RPA) pulmonary arteries, confirming that this vessel is the main pulmonary artery (MPA), consistent with transposition of the great arteries. Ao, Aorta. Reproduced from Reference [21].
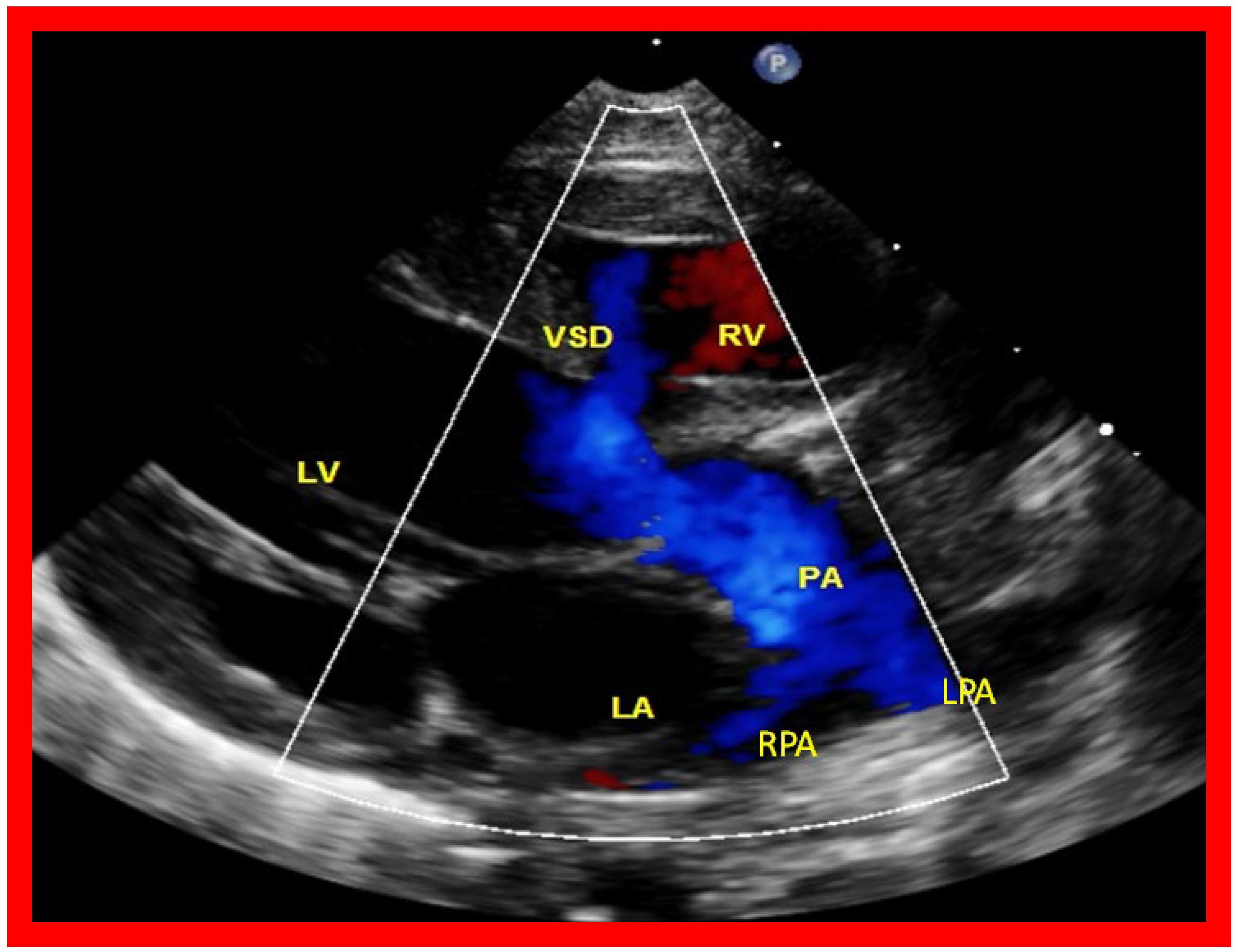
Figure 7. A selected video frame from a parasternal long axis view with color flow mapping of another patient with tricuspid atresia and transposition of the great arteries demonstrating the left atrium (LA), left ventricle (LV), a small right ventricle (RV) and a moderate sized ventricular septal defect (VSD). The vessel coming off the LV bifurcates into right (RPA) and left (LPA) pulmonary arteries. Reproduced from Reference [21]. PA, pulmonary artery.
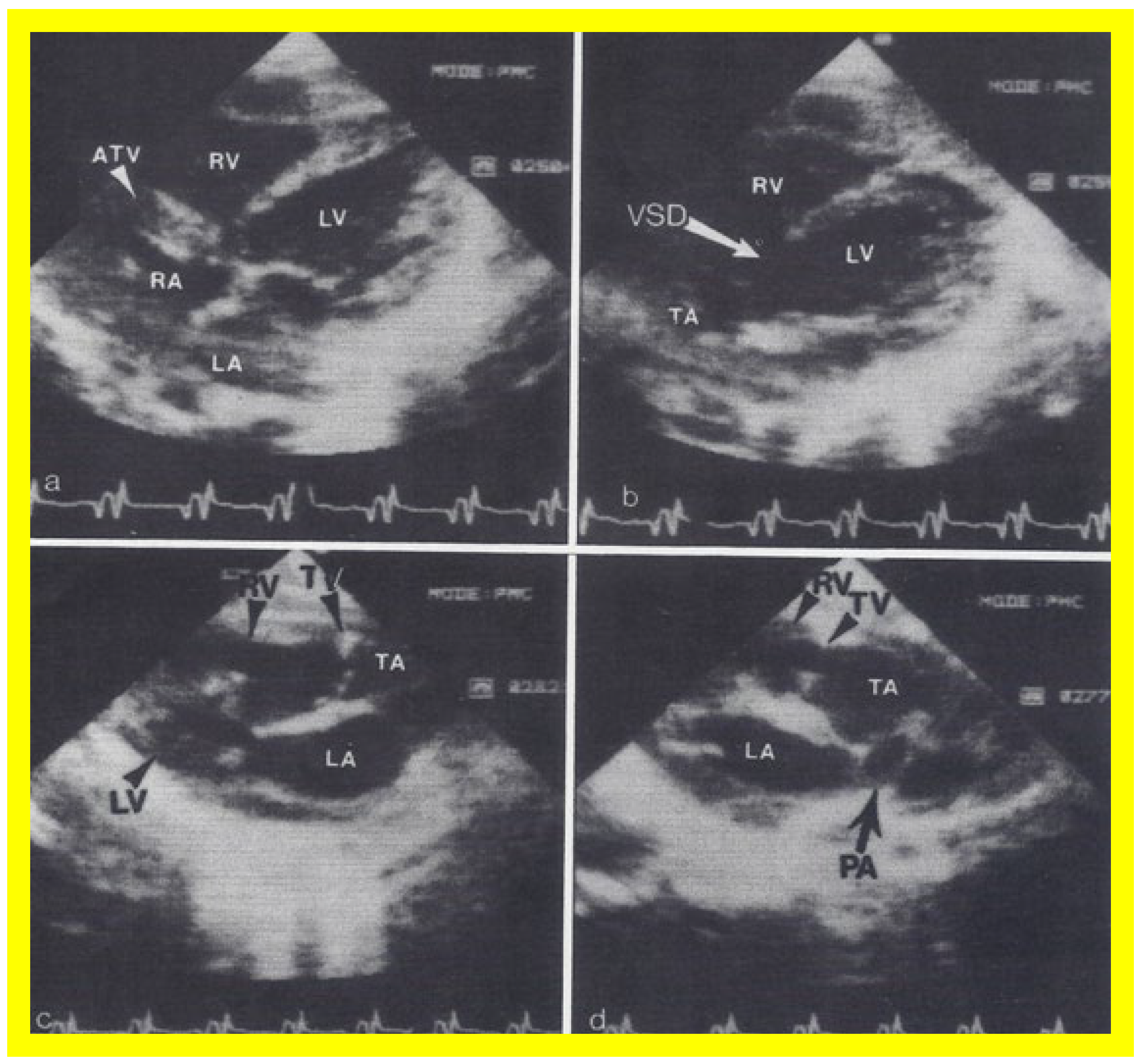
Figure 8. Two dimensional echocardiographic video frames demonstrating (a) an atretic tricuspid valve (ATV) between the right atrium (RA) and right ventricle (RV), (b) a large subtruncal ventricular septal defect (VSD), (c) thickened and somewhat domed truncal valve (TV) leaflets, and (d) the origin of the pulmonary artery (PA) from the posterior aspect of the truncus arteriosus (TA). LA, left atrium; LV, left ventricle. Reproduced from Rao PS, et al. Am Heart J 1991;122:829–835 [22].
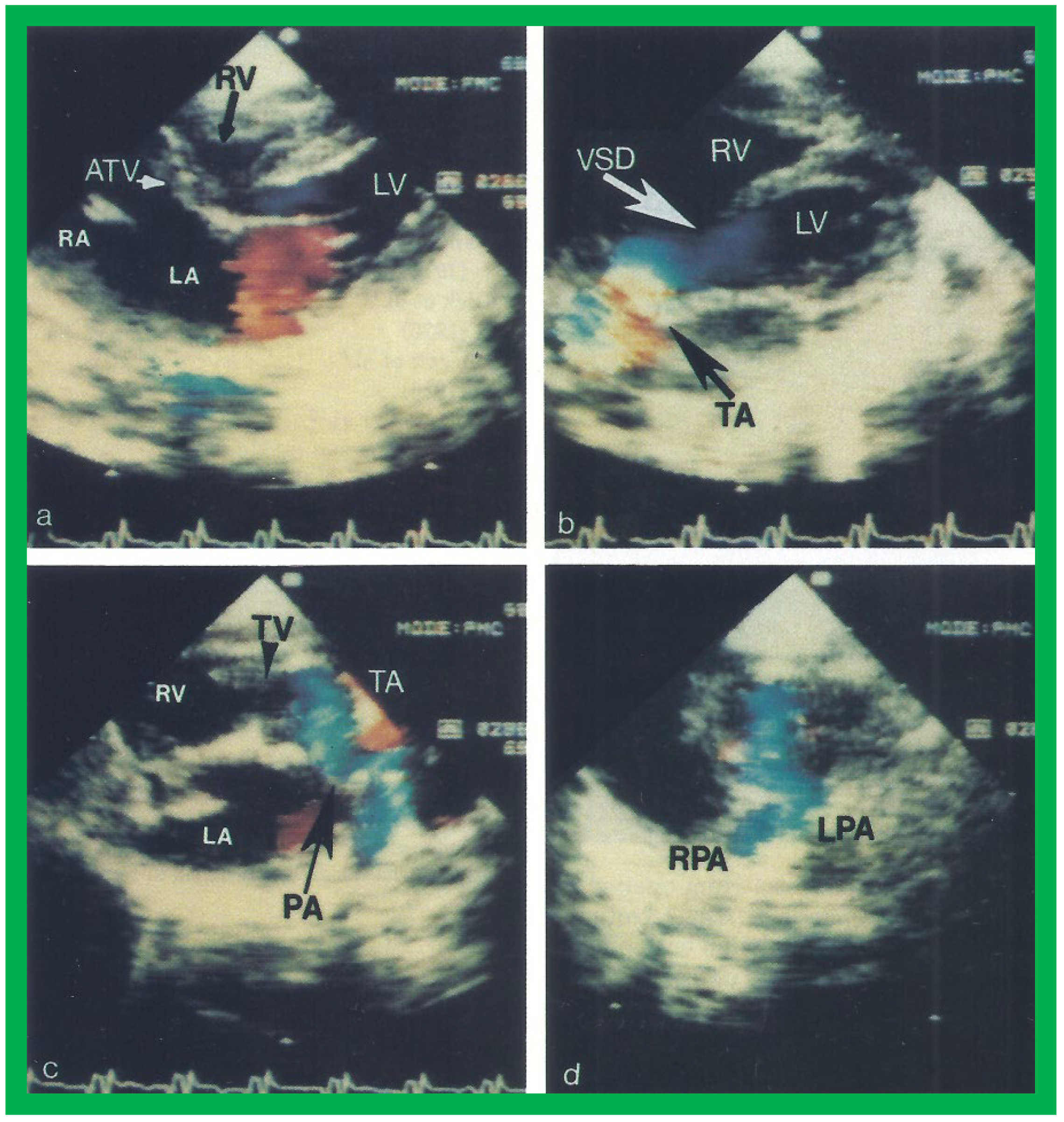
Figure 9. Video frames from a two dimensional echocardiographic and color Doppler study demonstrating (a) an atretic tricuspid valve (ATV) between the right atrium (RA) and right ventricle (RV), and blood flow from the left atrium (LA) into the left ventricle (LV) across the mitral valve. The RV (arrow) is very small and hypoplastic. (b) LV and RV with a large ventricular septal defect (VSD) below the truncus arteriosus (TA). Turbulent flow across the truncal valve suggests truncal valve stenosis. (c) The origin of the pulmonary artery (PA) from the TA by color flow (arrow), and (d) the division into right (RPA) and left (LPA) pulmonary arteries from the PA in a short-axis view. TV, truncal valve leaflets. Reproduced from Rao PS, et al. Am Heart J 1991;122:829–835 [22].
3. Summary and Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/app11209472
References
- Rao, P. A unified classification for tricuspid atresia. Am. Heart J. 1980, 99, 799–804.
- Rowe, R.D.; Freedom, R.M.; Mehrizi, A.; Bloom, K.R. The neonate with congenital heart disease. In Major Problems in Clinical Pediatrics, 2nd ed.; WB Saunders: Philadelphia, PA, USA, 1981; pp. 456–479.
- Vanpraagh, R.; Ando, M.; Dungan, W.T. Anatomic types of tricuspid atresia: Clinical and developmental implications (abstract). Circulation 1971, 44, 115.
- Astley, R.; Oldham, J.S.; Parsons, C. Congenital Tricuspid Atresia. Heart 1953, 15, 287–297.
- Müller, H.; Fall, I. Über zwei falle kongenitaler atreside des ostium venosum dextrum. Jahrb. Kinderh. Phys. Erzieh. 1906, 63, 235–249.
- Edwards, J.E.; Burchell, H.B. Congenital Tricuspid Atresia: A Classification. Med. Clin. N. Am. 1949, 33, 1177–1196.
- Keith, J.D.; Rowe, R.D.; Vlad, P. Tricuspid atresia. In Heart Disease in Infancy and Childhood; Macmillian: New York, NY, USA, 1958; pp. 434–470.
- Rao, P.S.; Alapati, S. Tricuspid atresia in the neonate. Neonatol. Today 2012, 7, 1–12.
- Rao, P.S. Tricuspid atresia: Anatomy, imaging and natural history. In Atlas of Heart Disease: Congenital Heart Disease; Freedom, R., Ed.; Current Medicine: Philadelphia, PA, USA, 1997.
- Rao, P.S. Classification of tricuspid atresia. In Tricuspid Atresia; Futura Publishing Co.: Mount Kisco, NY, USA, 1982; pp. 41–47.
- Rao, P.S.; Sissman, N.J. Spontaneous Closure of Physiologically Advantageous Ventricular Septal Defects. Circulation 1971, 43, 83–90.
- Rao, P.S. Natural history of the ventricular septal defect in tricuspid atresia and its surgical implications. Heart 1977, 39, 276–288.
- Rao, P.S. Further observations on the spontaneous closure of physiologically advantageous ventricular septal defects in tricuspid atresia: Surgical implications. Ann. Thorac. Surg. 1983, 35, 121–131.
- Gallaher, M.E.; Fyler, D.C. Observations on Changing Hemodynamics in Tricuspid Atresia without Associated Transposition of the Great Vessels. Circulation 1967, 35, 381–388.
- Sauer, U.; Hall, D. Spontaneous Closure or Critical Decrease in Size of the Ventricular Septal Defect in Tricuspid Atresia with Normally Connected Great Arteries: Surgical Implications. Herz 1981, 105–130.
- Rao, P.S. Physiologically advantageous ventricular septal defects (Letter). Pediatr. Cardiol. 1983, 4, 59–61.
- Rao, P. Natural history of ventricular septal defects in tricuspid atresia. In Tricuspid Atresia, 2nd ed.; Rao, P.S., Ed.; Futura Publishing Co.: Mount Kisco, NY, USA, 1992; pp. 261–293.
- Rao, P.S. Subaortic obstruction after pulmonary artery banding in patients with tricuspid atresia and double-inlet left ventricle and ventriculoarterial discordance (Letter). J. Am. Coll. Cardiol. 1991, 18, 1585–1586.
- Rao, P.S.; Covitz, W.; Chopra, P.S. Principles of palliative management of patients with tricuspid atresia. In Tricuspid Atresia, 2nd ed.; Rao, P.S., Ed.; Futura Publishing Co.: Mount Kisco, NY, USA, 1992; pp. 297–320.
- Rao, P.S. Atrioventricular canal mimicking tricuspid atresia: Echocardiographic and angiographic features. Heart 1987, 58, 409–412.
- Rao, P.S. Echocardiographic evaluation of neonates with suspected heart disease. In Perinatal Cardiology: A Multidisciplinary Approach; Rao, P.S., Vidyasagar, D., Eds.; Chapter 11; Cardiotext Publishing: Minneapolis, MN, USA, 2015.
- Rao, P.S.; Levy, J.; Nikicicz, E.; Gilbert-Barness, E.F. Tricuspid atresia: Association with persistent truncus arteriosus—A review. Am. Heart J. 1991, 122, 829–835.
