Mevalonate Kinase Deficiency (MKD) is a rare inborn disease belonging to the family of periodic fever syndromes. The MKD phenotype is characterized by systemic inflammation involving multiple organs, including the nervous system. Current anti-inflammatory approaches to MKD are only partially effective and do not act specifically on neural inflammation. According to the new emerging pharmacology trends, the repositioning of drugs from the indication for which they were originally intended to another one can make mechanistic-based medications easily available to treat rare diseases. According to this perspective, the squalene synthase inhibitor Lapaquistat (TAK-475), originally developed as a cholesterol-lowering drug, might find a new indication in MKD, by modulating the mevalonate cholesterol pathway, increasing the availability of anti-inflammatory isoprenoid intermediates.
- mevalonate
- inflammation
- drug repositioning
- rare disease
1. Introduction
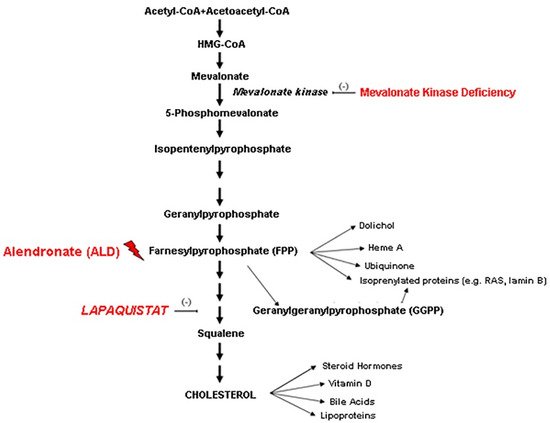
2. Discussion
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/biom11101438
References
- Buhaescu, I.; Izzedine, H. Mevalonate pathway: A review of clinical and therapeutical implications. Clin. Biochem. 2007, 40, 575–584.
- Drenth, J.P.; Cuisset, L.; Grateau, G.; Vasseur, C.; van de Velde-Visser, S.D.; de Jong, J.G.; Beckmann, J.S.; van der Meer, J.W.; Delpech, M. Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. International Hyper-IgD Study Group. Nat. Genet. 1999, 22, 178–181.
- Houten, S.M.; van Woerden, C.S.; Wijburg, F.A.; Wanders, R.J.; Waterham, H.R. Carrier frequency of the V377I (1129G>A) MVK mutation, associated with Hyper-IgD and periodic fever syndrome, in the Netherlands. Eur. J. Hum. Genet. 2003, 11, 196–200.
- Hoffmann, G.; Gibson, K.M.; Brandt, I.K.; Bader, P.I.; Wappner, R.S.; Sweetman, L. Mevalonic aciduria—An inborn error of cholesterol and nonsterol isoprene biosynthesis. N. Engl. J. Med. 1986, 314, 1610–1614.
- Akula, M.K.; Shi, M.; Jiang, Z.; Foster, C.E.; Miao, D.; Li, A.S.; Zhang, X.; Gavin, R.M.; Forde, S.D.; Germain, G.; et al. Control of the innate immune response by the mevalonate pathway. Nat. Immunol. 2016, 17, 922–929.
- Park, Y.H.; Wood, G.; Kastner, D.L.; Chae, J.J. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat. Immunol. 2016, 17, 914–921.
- Ibrahim, J.N.; Jéru, I.; Lecron, J.C.; Medlej-Hashim, M. Cytokine signatures in hereditary fever syndromes (HFS). Cytokine Growth Factor Rev. 2017, 33, 19–34.
- Marcuzzi, A.; Zanin, V.; Kleiner, G.; Monasta, L.; Crovella, S. Mouse model of mevalonate kinase deficiency: Comparison of cytokine and chemokine profile with that of human patients. Pediatr. Res. 2013, 74, 266–271.
- Marcuzzi, A.; Piscianz, E.; Vecchi Brumatti, L.; Tommasini, A. Mevalonate kinase deficiency: Therapeutic targets, treatments, and outcomes. Expert Opin. Orphan Drugs 2017, 5, 515–524.
- Marcuzzi, A.; Loganes, C.; Valencic, E.; Piscianz, E.; Monasta, L.; Bilel, S.; Bortul, R.; Celeghini, C.; Zweyer, M.; Tommasini, A. Neuronal Dysfunction Associated with Cholesterol Deregulation. Int. J. Mol. Sci. 2018, 19, 1523.
- De Benedetti, F.; Gattorno, M.; Anton, J.; Ben-Chetrit, E.; Frenkel, J.; Hoffman, H.M.; Koné-Paut, I.; Lachmann, H.J.; Ozen, S.; Simon, A.; et al. Canakinumab for the Treatment of Autoinflammatory Recurrent Fever Syndromes. N. Engl. J. Med. 2018, 378, 1908–1919.
- Malcova, H.; Strizova, Z.; Milota, T.; Striz, I.; Sediva, A.; Cebecauerova, D.; Horvath, R. IL-1 Inhibitors in the Treatment of Monogenic Periodic Fever Syndromes: From the Past to the Future Perspectives. Front. Immunol. 2021, 11, 619257.
- Schneiders, M.S.; Houten, S.M.; Turkenburg, M.; Wanders, R.J.; Waterham, H.R. Manipulation of isoprenoid biosynthesis as a possible therapeutic option in mevalonate kinase deficiency. Arthritis Rheum. 2006, 54, 2306–2313.
- Nishimoto, T.; Amano, Y.; Tozawa, R.; Ishikawa, E.; Imura, Y.; Yukimasa, H.; Sugiyama, Y. Lipid-lowering properties of TAK-475, a squalene synthase inhibitor, in vivo and in vitro. Br. J. Pharmacol. 2003, 139, 911–918.
- Amano, Y.; Nishimoto, T.; Tozawa, R.; Ishikawa, E.; Imura, Y.; Sugiyama, Y. Lipid-lowering effects of TAK-475, a squalene synthase inhibitor, in animal models of familial hypercholesterolemia. Eur. J. Pharmacol. 2003, 466, 155–161.
- Henneman, L.; van Cruchten, A.G.; Kulik, W.; Waterham, H.R. Inhibition of the isoprenoid biosynthesis pathway; detection of intermediates by UPLC-MS/MS. Biochim. Biophys. Acta 2011, 1811, 227–233.
- Shidoji, Y.; Tabata, Y. Unequivocal evidence for endogenous geranylgeranoic acid biosynthesized from mevalonate in mammalian cells. J. Lipid Res. 2019, 60, 579–593.
- Marcuzzi, A.; Piscianz, E.; Zweyer, M.; Bortul, R.; Loganes, C.; Girardelli, M.; Baj, G.; Monasta, L.; Celeghini, C. Geranylgeraniol and Neurological Impairment: Involvement of Apoptosis and Mitochondrial Morphology. Int. J. Mol. Sci. 2016, 17, 365.
- Marcuzzi, A.; Loganes, C.; Celeghini, C.; Kleiner, G. Repositioning Of Tak-475 in Mevalonate Kinase Disease: Translating Theory into Practice. Curr. Med. Chem. 2018, 25, 2783–2796.
- Ebihara, T.; Teshima, K.; Kondo, T.; Tagawa, Y.; Moriwaki, T.; Asahi, S. Pharmacokinetics of TAK-475, a Squalene Synthase Inhibitor, in Rats and Dogs. Drug Res. 2016, 66, 287–292.
- Seiki, S.; Frishman, W.H. Pharmacologic inhibition of squalene synthase and other downstream enzymes of the cholesterol synthesis pathway: A new therapeutic approach to treatment of hypercholesterolemia. Cardiol. Rev. 2009, 17, 70–76.
- Liao, J.K. Squalene synthase inhibitor lapaquistat acetate: Could anything be better than statins? Circulation 2011, 123, 1925–1928.
- Martorana, D.; Bonatti, F.; Mozzoni, P.; Vaglio, A.; Percesepe, A. Monogenic Autoinflammatory Diseases with Mendelian Inheritance: Genes, Mutations, and Genotype/Phenotype Correlations. Front. Immunol. 2017, 8, 344.
- Jeyaratnam, J.; Frenkel, J. Management of Mevalonate Kinase Deficiency: A Pediatric Perspective. Front Immunol. 2020, 11, 1150.
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388.
- Bao, M.; Yi, Z.; Fu, Y. Activation of TLR7 Inhibition of Mycobacterium Tuberculosis Survival by Autophagy in RAW 264.7 Macrophages. J. Cell. Biochem. 2017, 118, 4222–4229.
- Simon, A.; Drewe, E.; van der Meer, J.W.; Powell, R.J.; Kelley, R.I.; Stalenhoef, A.F.; Drenth, J.P. Simvastatin treatment for inflammatory attacks of the hyperimmunoglobulinemia D and periodic fever syndrome. Clin. Pharmacol. Ther. 2004, 75, 476–483.
- Hoffmann, G.F.; Charpentier, C.; Mayatepek, E.; Mancini, J.; Leichsenring, M.; Gibson, K.M.; Divry, P.; Hrebicek, M.; Lehnert, W.; Sartor, K.; et al. Clinical and biochemical phenotype in 11 patients with mevalonic aciduria. Pediatrics 1993, 91, 915–921.
- Hübner, C.; Hoffmann, G.F.; Charpentier, C.; Gibson, K.M.; Finckh, B.; Puhl, H.; Lehr, H.A.; Kohlschütter, A. Decreased plasma ubiquinone-10 concentration in patients with mevalonate kinase deficiency. Pediatr. Res. 1993, 34, 129–133.
- Olsen, B.N.; Schlesinger, P.H.; Ory, D.S.; Baker, N.A. 25-Hydroxycholesterol increases the availability of cholesterol in phospholipid membranes. Biophys. J. 2011, 100, 948–956.
- Finsterer, J.; Frank, M. Repurposed drugs in metabolic disorders. Curr. Top. Med. Chem. 2013, 13, 2386–2394.
- Lotfi Shahreza, M.; Ghadiri, N.; Mousavi, S.R.; Varshosaz, J.; Green, J.R. A review of network-based approaches to drug repositioning. Brief. Bioinform. 2018, 19, 878–892.
- Suzuki, N.; Ito, T.; Matsui, H.; Takizawa, M. Anti-inflammatory and cytoprotective effects of a squalene synthase inhibitor, TAK-475 active metabolite-I, in immune cells simulating mevalonate kinase deficiency (MKD)-like condition. Springerplus 2016, 5, 1429.
- Maryam Lotfi Shahreza; Nasser Ghadiri; Sayed Rasoul Mousavi; Jaleh Varshosaz; James Green; A review of network-based approaches to drug repositioning. Briefings in Bioinformatics 2017, 19, 878-892, 10.1093/bib/bbx017.
- Nobutaka Suzuki; Tatsuo Ito; Hisanori Matsui; Masayuki Takizawa; Anti-inflammatory and cytoprotective effects of a squalene synthase inhibitor, TAK-475 active metabolite-I, in immune cells simulating mevalonate kinase deficiency (MKD)-like condition. SpringerPlus 2016, 5, 1429-1429, 10.1186/s40064-016-3125-1.
