With the increasing interest in obtaining biologically active compounds from natural sources, Dittrichia viscosa (L.) Greuter (Asteraceae) came into our focus as a readily available and aromatic wild shrub widely distributed in the Mediterranean region. This work provides a phytochemical profile of D. viscosa in terms of parallel chemical composition in the lipophilic fraction (essential oil) and the water fraction (hydrosol). GC-MS analysis identified 1,8-cineole, caryophyllene oxide, α-terpenyl acetate, and α-muurolol as the major components of the essential oil, while in the hydrosol p-menth-1-en-9-ol, 1,8-cineole, linalool, cis-sabinene hydrate, and α-muurolol were the major volatile components. 3,4-Dihydroxybenzoic acid was found to be the predominant compound in the hydrosol composition by HPLC analysis. The antimicrobial potential of both extracts was evaluated against thirteen opportunistic pathogens associated with common skin and wound infections and emerging food spoilage microorganisms. The antimicrobial activity of the essential oil suggests that the volatiles of D. viscosa could be used as novel antimicrobial agents.
- Dittrichia viscosa
- essential oil
- hydrosol
- GSH
- antimicrobial
- antiproliferative
- antiphytoviral activity
1. Introduction
2. Current Results and Discussion
2.1. Gas Chromatography and Mass Spectrometry (GC-MS) Analysis of the Free Volatile Compounds from Essential Oil and Hydrosol
| Component | RI* | RI** | EO (Yield in %) |
H (Yield in %) |
|---|---|---|---|---|
| Monoterpene hydrocarbons | 0.71 | 1.56 | ||
| α-Pinene * | 938 | 1036 | 0.71 ± 0.01 a | 0.23 ± 0.01 b |
| Sabinene | 971 | 1115 | - | 0.28 ± 0.01 |
| Myrcene | 992 | 1145 | - | 0.62 ± 0.03 |
| Limonene | 1032 | 1204 | - | 0.43 ± 0.03 |
| Oxygenated monoterpenes | 53.41 | 81.85 | ||
| 1,8-Cineole | 1030 | 1211 | 16.41 ± 0.01 b | 18.55 ± 0.01 a |
| cis-Sabinene hydrate | 1065 | 1561 | 4.23 ± 0.01 b | 10.97 ± 0.01 a |
| Linalool | 1099 | 1548 | 6.62 ± 0.01 b | 11.67 ± 0.01 a |
| Borneol * | 1176 | 1699 | 0.32 ± 0.01 a | 0.12 ± 0.05 b |
| α-Terpineol | 1186 | 1646 | 2.65 ± 0.01 a | 2.62 ± 0.01 b |
| β-Cyclocitral | 1223 | 1629 | 4.81 ± 0.01 a | 2.23 ± 0.01 b |
| Bornyl acetate | 1287 | 1591 | 2.71 ± 0.01 a | 0.67 ± 0.01 b |
| p-Menth-1-en-9-ol | 1294 | 1915 | - | 29.93 ± 0.01 |
| α-Terpinyl acetate | 1349 | 1685 | 13.92 ± 0.01 a | 1.31 ± 0.01 b |
| Cyclohexene, 1,5,5-trimethyl-6-methylene | 1364 | - | - | 2.02 ± 0.01 |
| (E)-Isoeugenol | 1446 | 2314 | 1.74 ± 0.01 | 1.76 ± 0.01 |
| Sesquiterpene hydrocarbons | 7.26 | - | ||
| allo-Aromadendrene | 1465 | 1662 | 2.34 ± 0.01 | - |
| β-Bisabolene | 1494 | 1729 | 4.31 ± 0.01 | - |
| δ-Cadinene | 1517 | 1754 | 0.61 ± 0.07 | - |
| Oxygenated sesquiterpenes | 30.11 | 13.49 | ||
| Caryophyllene-oxide * | 1581 | 1955 | 15.14 ± 0.01 a | 3.24 ± 0.01 b |
| α-Muurolol | 1645 | 2181 | 13.75 ± 0.01 a | 10.25 ± 0.01 b |
| Cyperotundone | 1696 | - | 1.22 ± 0.01 | - |
| Fatty acids | 2.58 | - | ||
| Hexadecanoic acid | 1959 | 2913 | 2.58 ± 0.01 | - |
| Hydrocarbons | 2.67 | - | ||
| Heneicosane * | 2100 | 2100 | 0.42 ± 0.03 | - |
| Docosane * | 2200 | 2200 | 1.73 ± 0.01 | - |
| Tricosane * | 2300 | 2300 | 0.52 ± 0.01 | - |
| Total identification (%) | 96.74 | 96.90 |
2.2. HPLC Analysis of Hydrosol
| Phenolic Compound | mg/L ± SD |
|---|---|
| 3,4-dihydroxybenzoic acid | 62.24 ± 2.72 |
| caffeic acid | 0.90 ± 0.06 |
| trans-o-coumaric acid | 0.39 ± 0.02 |
| cinnamic acid | 1.16 ± 0.01 |
| luteolin | 1.75 ± 0.13 |
2.3. Wide-Spectrum Antimicrobial Activity
| Species | Strain Origin | Essential Oil (mg/mL) a | |
|---|---|---|---|
| MIC | MBC | ||
| Gram-positive bacteria | |||
| Staphylococcus aureus | ATCC 29213 | 2.8 | 2.8 |
| Staphylococcus aureus | Clinical/MRSA | 5.6 | 5.6 |
| Staphylococcus epidermidis | Human | 1.4 | 1.4 |
| Streptococcus pyogenes | ATCC 19615 | 0.09 | 0.09 |
| Streptococcus agalactiae | Clinical | 0.09 | 0.09 |
| Enterococcus faecalis | ATCC 29212 | 1.4 | 2.8 |
| Listeria monocytogenes | ATCC 19111 (1/2a) | 2.8 | 2.8 |
| Bacillus cereus | Food | 0.7 | 0.7 |
| Clostridium perfringens | Food | 0.09 | 0.09 |
| Gram-negative bacteria | |||
| Escherichia coli | ATCC 25922 | 2.8 | 2.8 |
| Acinetobacter baumannii | ATCC 19606 | 5.6 | 5.6 |
| Yeast | MIC50 | MIC90 | |
| Candida albicans | ATCC 90029 | 2.8 | 5.6 |
| Molds | MIC50 | MIC90 | |
| Aspergillus niger | Food | 0.09 | 5.6 |
2.4. Antiproliferative Activity
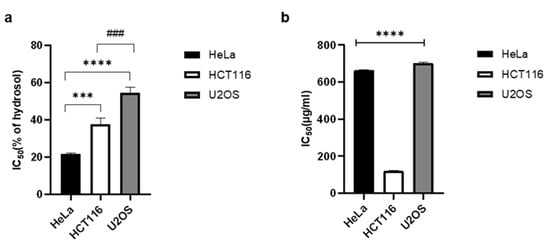
2.5. Glutathione (GSH) Assay
| Control | H Treated | |
|---|---|---|
| GSH level | 0.287 ± 0.007 a | 0.187 ± 0.025 b |
2.6. Antiphytoviral Activity
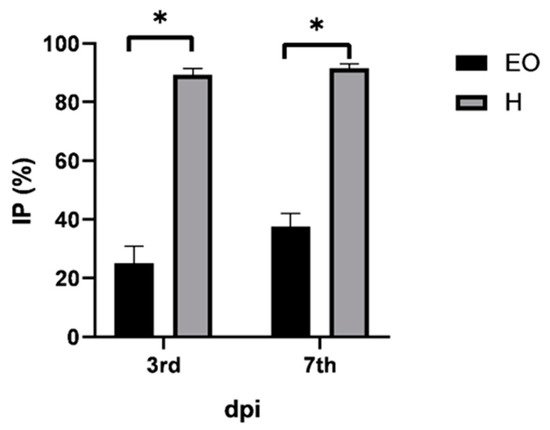
| dpi | LLN ± SD | |
|---|---|---|
| 3rd | C | 6.2 ± 0.2 a |
| EO | 4.6 ± 0.2 b | |
| H | 0.7 ± 0.2 c | |
| 7th | C | 14.2 ± 2.4 a |
| EO | 8.9 ± 2.0 b | |
| H | 1.2 ± 0.4 c | |
This entry is adapted from the peer-reviewed paper 10.3390/plants10091837
References
- Zaki, M.; Loubidi, M.; Oukhrib, A.; Mallouk, S. Natural products from Dittrichia Viscosa (Mini-Review). RHAZES Green Appl. Chem. 2019, 9, 30–46.
- Maleš, Ž.; Šarić, F. Kvantitativna analiza fenolnih spojeva ljepljivog omana-Inula viscosa (L.) Ait. Farm. Glas. 2009, 65, 143–148.
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, 2nd ed.; Cambridge University Press: London, UK, 1964–1980; Volume 1–5.
- Pignatti, S. Flora Ditalia; Edagricole: Bologna, Italy, 1982; Volume I–III.
- Flora Croatica Database: 26865. Available online: https://hirc.botanic.hr/fcd/DetaljiFrame.aspx?IdVrste=26865&taxon=Dittrichia+viscosa+(L.)+Greuter (accessed on 20 July 2021).
- Moeini, A.; van Reenen, A.; Van Otterlo, W.; Cimmino, A.; Masi, M.; Lavermicocca, P.; Valerio, F.; Immirzi, B.; Santagata, G.; Malinconico, M.; et al. α-costic acid, a plant sesquiterpenoid from Dittrichia viscosa, as modifier of Poly (lactic acid) properties: A novel exploitation of the autochthone biomass metabolite for a wholly biodegradable system. Ind. Crops Prod. 2020, 146, 112134.
- Barrero, A.F.; Herrador, M.M.; Arteaga, P.; Catalán, J.V. Dittrichia viscosa L. Greuter: Phytochemistry and biological activity. Nat. Prod. Commun. 2008, 3, 1799–1804.
- Zouaghi, N.; El, N.; Bensiradj, H.; Cavaleiro, C.; Nadjemi, B.; Telfah, A. Antimicrobial activities of natural volatiles organic compounds extracted from Dittrichia viscosa (L.) by hydrodistillation. Jordan J. Biol. Sci 2021, 14, 41–49.
- Eljazi, J.S.; Selmi, S.; Zarroug, Y.; Wesleti, I.; Aouini, B.; Jallouli, S.; Limam, F. Essential oil composition, phenolic compound, and antioxidant potential of Inulaviscosa as affected by extraction process. Int. J. Food Prop. 2018, 21, 2309–2319.
- Haoui, I.E.; Derriche, R.; Madani, L.; Oukali, Z. Analysis of the chemical composition of essential oil from Algerian Inula viscosa (L.) Aiton. Arab. J. Chem. 2015, 8, 587–590.
- Grauso, L.; Cesarano, G.; Zotti, M.; Ranesi, M.; Sun, W.; Bonanomi, G.; Lanzotti, V. Exploring Dittrichia viscosa (L.) Greuter phytochemical diversity to explain its antimicrobial, nematicidal and insecticidal activity. Phytochem. Rev. 2020, 19, 659–689.
- Hamedi, A.; Pasdaran, A.; Zebarjad, Z.; Moein, M. A survey on chemical constituents and indications of aromatic waters soft drinks (Hydrosols) used in Persian nutrition culture and folk medicine for neurological disorders and mental health. J. Evid. Based Complementary Altern. Med. 2017, 22, 744–752.
- Rhimi, W.; Ben Salem, I.; Iatta, R.; Chaabane, H.; Saidi, M.; Boulila, A.; Cafarchia, C. Dittrichia viscosa L. leaves lipid extract: An unexploited source of essential fatty acids and tocopherols with antifungal and anti-inflammatory properties. Ind. Crops Prod. 2018, 113, 196–201.
- Bedoya, L.M.; Sanchez Palomino, S.; Abad, M.J.; Bermejo, P.; Alcami, J. Screening of selected plant extracts for in vitro inhibitory activity on human immunodeficiency virus. Phyther. Res. 2002, 16, 550–554.
- Yaniv, Z.; Dafni, A.; Friedman, J.; Palevitch, D. Plants used for the treatment of diabetes in Israel. J. Ethnopharmacol. 1987, 19, 145–151.
- Mohti, H.; Taviano, M.F.; Cacciola, F.; Dugo, P.; Mondello, L.; Marino, A.; Crisafi, G.; Benameur, Q.; Zaid, A.; Miceli, N. Inula viscosa (L.) Aiton leaves and flower buds: Effect of extraction solvent/technique on their antioxidant ability, antimicrobial properties and phenolic profile. Nat. Prod. Res. 2020, 34, 46–52.
- Ozkan, E.; Karakas, F.P.; Yildirim, A.B.B.; Tas, I.; Eker, I.; Yavuz, M.Z.; Turker, A.U. Promising medicinal plant Inula viscosa L.: Antiproliferative, antioxidant, antibacterial and phenolic profiles. Prog. Nutr. 2019, 21, 652–661.
- Messaoudi, M.; Chahmi, N.; El Mzibri, M.; Gmouh, S.; Amzazi, S.; Benbacer, L.; El Hassouni, M. Cytotoxic effect and chemical composition of Inula viscosa from three different regions of morocco. Eur. J. Med. Plants 2016, 16, 1–9.
- Benbacer, L.; Merghoub, N.; El Btaouri, H.; Gmouh, S.; Attaleb, M.; Morjani, H.; Amzazi, S.; Mzibri, M. Antiproliferative effect and induction of apoptosis by Inula viscosa L. and Retama monosperma L. Extracts in human cervical cancer cells. In Topics on Cervical Cancer with an Advocacy for Prevention; IntechOpen: London, UK, 2012.
- Rozenblat, S.; Grossman, S.; Bergman, M.; Gottlieb, H.; Cohen, Y.; Dovrat, S. Induction of G2/M arrest and apoptosis by sesquiterpene lactones in human melanoma cell lines. Biochem. Pharmacol. 2007, 75, 369–382.
- Dunkic, V.; Bezic, N.; Vuko, E.; Cukrov, D. Antiphytoviral activity of Satureja montana L. ssp. variegata (host) P.W. Ball essential oil and phenol compounds on CMV and TMV. Molecules 2010, 15, 6713–6721.
- Dunkić, V.; Bezić, N.; Vuko, E. Antiphytoviral activity of essential oil from endemic species Teucrium arduini. Nat. Prod. Commun. 2011, 6, 1385–1388.
- Dunkić, V.; Vuko, E.; Bezić, N.; Kremer, D.; Ruščić, M. Composition and antiviral activity of the essential oils of Eryngium alpinum and E. amethystinum. Chem. Biodivers. 2013, 10, 1894–1902.
- Bezić, N.; Vuko, E.; Dunkić, V.; Ruščić, M.; Blažević, I.; Burčul, F. Antiphytoviral activity of sesquiterpene-rich essential oils from four croatian teucrium species. Molecules 2011, 16, 8119–8129.
- Vuko, E.; Rusak, G.; Dunkić, V.; Kremer, D.; Kosalec, I.; Rađa, B.; Bezić, N. Inhibition of Satellite RNA Associated Cucumber Mosaic Virus Infection by Essential Oil of Micromeria croatica (Pers.) Schott. Molecules 2019, 24, 1342.
- Vuko, E.; Dunkić, V.; Ruščić, M.; Nazlić, M.; Mandić, N.; Soldo, B.; Šprung, M.; Fredotović, Ž. Chemical composition and new biological activities of essential oil and hydrosol of Hypericum perforatum L. ssp. veronense (Schrank) H. Lindb. Plants 2021, 10, 1014.
- Vuko, E.; Dunkić, V.; Bezić, N.; Ruščić, M.; Kremer, D. Chemical composition and antiphytoviral activity of essential oil of Micromeria graeca. Nat. Prod. Commun. 2012, 7, 1227–1230.
- Lu, M.; Han, Z.; Xu, Y.; Yao, L. In vitro and in vivo anti-tobacco mosaic virus activities of essential oils and individual compounds. J. Microbiol. Biotechnol. 2013, 23, 771–778.
- Bishop, C.D. Antiviral activity of the essential oil of melaleuca alternifolia (Maiden amp; Betche) cheel (tea tree) against tobacco mosaic virus. J. Essent. Oil Res. 1995, 7, 641–644.
- Madani, L.; Derriche, R.; Haoui, I.E. Essential oil of Algerian Inulaa viscosa leaves. J. Essent. Oil Bear. Plants 2014, 17, 164–168.
- Gharred, N.; Dbeibia, A.; Falconieri, D.; Hammami, S.; Piras, A.; Dridi-Dhaouadi, S. Chemical composition, antibacterial and antioxidant activities of essential oils from flowers, leaves and aerial parts of Tunisian Dittrichia Viscosa. J. Essent. Oil Res. 2019, 31, 582–589.
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4.1 ed.; Allured Publishing: Carol Stream, IL, USA, 2017.
- Nazlić, M.; Kremer, D.; Grubešić, R.J.; Soldo, B.; Vuko, E.; Stabentheiner, E.; Ballian, D.; Bogunić, F.; Dunkić, V. Endemic Veronica saturejoides vis. ssp. saturejoides–chemical composition and antioxidant activity of free volatile compounds. Plants 2020, 9, 1646.
- Beara, I.; Živković, J.; Lesjak, M.; Ristić, J.; Šavikin, K.; Maksimović, Z.; Janković, T. Phenolic profile and anti-inflammatory activity of three Veronica species. Ind. Crops Prod. 2015, 63, 276–280.
- Stojković, D.S.; Živkovic, J.; Soković, M.; Glamočlija, J.; Ferreira, I.C.; Janković, T.; Maksimović, Z. Antibacterial activity of Veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem. Toxicol. 2013, 55, 209–213.
- Cid-Pérez, T.S.; Ávila-Sosa, R.; Ochoa-Velasco, C.E.; Rivera-Chavira, B.E.; Nevárez-Moorillón, G.V. Antioxidant and antimicrobial activity of mexican oregano (Poliomintha longiflora) essential oil, hydrosol and extracts fromwaste solid residues. Plants 2019, 8, 22.
- Li, X.; Wang, X.; Chen, D.; Chen, S. Antioxidant activity and mechanism of protocatechuic acid in vitro. Funct. Foods Health Dis. 2011, 1, 232–244.
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules 2020, 10, 221.
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128.
- Perfect, J.R.; Cox, G.M.; Lee, J.Y.; Kauffman, C.A.; de Repentigny, L.; Chapman, S.W.; Morrison, V.A.; Pappas, P.; Hiemenz, J.W.; Stevens, D.A.; et al. The impact of culture isolation of aspergillus species: A hospital-based survey of aspergillosis. Clin. Infect. Dis. 2001, 33, 1824–1833.
- Ki, V.; Rotstein, C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can. J. Infect. Dis. Med Microbiol. 2008, 19, 173–184.
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641.
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582.
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti. Infect. Ther. 2013, 11, 297–308.
- Ali-Shtayeh, M.S.; Yaghmour, R.M.; Faidi, Y.R.; Salem, K.; Al-Nuri, M.A. Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. J. Ethnopharmacol. 1998, 60, 265–271.
- Al-Masri, M.I.; Sharawi, S.M.; Barakat, R.M.; Al-Masri, M.I.; Sharawi, S.M.; Barakat, R.M. Effect of clammy inula (inula viscose) plant extract in combination with a low dose of the fungicide iprodione on botrytis cinerea in vitro and in vivo. Am. J. Plant Sci. 2015, 6, 1519–1526.
- Blanc, M.C.; Bradesi, P.; Gonçalves, M.J.; Salgueiro, L.; Casanova, J. Essential oil of Dittrichia viscosa ssp. viscosa: Analysis by 13C-NMR and antimicrobial activity. Flavour Fragr. J. 2006, 21, 324–332.
- Bonsignore, L.; Loy, G.; Secci, D.; Logu, A.; de Palmieri, G. A preliminary microbiological screening of Sardinian plants. Fitoterapia 1990, 61, 339–341.
- Bekkara, F.; Benhammou, N.; Panovska, T.K. Biological activities of the essential oil and ethanolic extract of Inula viscosa from the Tlemcen region of Algeria. Adv. Food Sci. 2008, 30, 132–139.
- Talib, W.H.; Mahasneh, A.M. Antiproliferative activity of plant extracts used against cancer in traditional medicine. Sci. Pharm. 2010, 78, 33.
- Çelik, T.A.; Aslantürk, Ö.S. Evaluation of cytotoxicity and genotoxicity of Inula viscosa leaf extracts with Allium test. J. Biomed. Biotechnol. 2010, 2010, 189252.
- Kaileh, M.; Vanden Berge, W.; Boone, E.; Essawi, T.; Haegman, G. Screening of indigenous Palestinian medicinal plants for potential anti-inflammatory and cytotoxic activity. J. Ethnopharmacol. 2007, 113, 510–516.
- Merghoub, N.; El Btaouri, H.; Benbacer, L.; Gmouth, S.; Trentesaux, C.; Brassart, B.; Terryn, C.; Attaleb, M.; Madoulet, C.; Benjouad, A.; et al. Inula viscosa extracts induces telomere shortening and apoptosis in cancer cells and overcome drug resistance. Nutr. Cancer 2016, 68, 131–143.
- Talib, W.H.; Mahasneh, A.M. Antimicrobial, cytotoxicity and phytochemical screening of Jordanian plants used in traditional medicine. Molecules 2010, 15, 1811–1824.
- Afifi-Yazar, F.U.; Kasabri, V.; Abu-Dahab, R. Medicinal plants from Jordan in the treatment of cancer: Traditional uses vs. in vitro and in vivo evaluations—Part 1. Planta Med. 2011, 77, 1203–1209.
- Talib, W.H.; Abu Zarga, M.H.; Mahasneh, A.M. Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from Inula viscosa. Molecules 2012, 17, 3291–3303.
- Seca, A.M.L.; Grigore, A.; Pinto, D.C.G.A.; Silva, A.M.S. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J. Ethnopharmacol. 2014, 154, 286–310.
- Zeouk, I.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Bethencourt-Estrella, C.J.; Bazzocchi, I.L.; Bekhti, K.; Lorenzo-Morales, J.; Jiménez, I.A.; Piñero, J.E. Sesquiterpenoids and flavonoids from Inula viscosa induce programmed cell death in kinetoplastids. Biomed. Pharmacother. 2020, 130, 110518.
- Asakawa, Y. Dietary monoterpenoids. In Handbook of Dietary Phytochemicals; Springer: Singapore, 2021; pp. 607–731.
- Moteki, H.; Hibasami, H.; Yamada, Y.; Katsuzaki, H.; Imai, K.; Komiya, T. Specific induction of apoptosis by 1,8-cineole in two human leukemia cell lines, but not a in human stomach cancer cell line. Oncol. Rep. 2002, 9, 757–760.
- Murata, S.; Shiragami, R.; Kosugi, C.; Tezuka, T.; Yamazaki, M.; Hirano, A.; Yoshimura, Y.; Suzuki, M.; Shuto, K.; Ohkohchi, N.; et al. Antitumor effect of 1, 8-cineole against colon cancer. Oncol. Rep. 2013, 30, 2647–2652.
- Abdalla, A.N.; Shaheen, U.; Abdallah, Q.M.A.; Flamini, G.; Bkhaitan, M.M.; Abdelhady, M.I.S.; Ascrizzi, R.; Bader, A. Proapoptotic activity of achillea membranacea essential oil and its major constituent 1,8-cineole against a2780 ovarian cancer cells. Molecules 2020, 25, 1582.
- Anter, J.; Romero-Jiménez, M.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Analla, M.; Alonso-Moraga, Á.; Muñoz-Serrano, A. Antigenotoxicity, cytotoxicity, and apoptosis induction by apigenin, bisabolol, and protocatechuic acid. J. Med. Food 2011, 14, 276–283.
- Kan, A.K.; Rehana, R.; Fatima, N.; Mahmood, S.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological activities of protocatechuic acid. Acta Pol. Pharm. 2015, 72, 643–650.
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.J.; Gescher, A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Prev. Biomark. 2000, 9, 1163–1170.
- Lee, I.R.; Yang, M.Y. Phenolic compounds from Duchesnea chrysantha and their cytotoxic activities in human cancer cell. Arch. Pharm. Res. 1994, 17, 476–479.
- Tseng, T.H.; Kao, T.W.; Chu, C.Y.; Chou, F.P.; Lin, W.L.; Wang, C.J. Induction of apoptosis by hibiscus protocatechuic acid in human leukemia cells via reduction of retinoblastoma (RB) phosphorylation and Bcl-2 expression. Biochem. Pharmacol. 2000, 60, 307–315.
- Kampa, M.; Alexaki, V.-I.; Notas, G.; Nifli, A.-P.; Nistikaki, A.; Hatzoglou, A.; Bakogeorgou, E.; Kouimtzoglou, E.; Blekas, G.; Boskou, D.; et al. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action. Breast Cancer Res. 2004, 6, R63.
- Yip, E.C.; Chan, A.S.; Pang, H.; Tam, Y.K.; Wong, Y.H. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biol. Toxicol. 2006, 22, 293–302.
- Klauning, J.E.; Kamendulis, L.M. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 239–267.
- Tanaka, T.; Tanaka, T.; Tanaka, M. Potential cancer chemopreventive activity of protocatechuic acid. J. Exp. Clin. Med. 2011, 3, 27–33.
- Ortega, A.L.; Mena, S.; Estrela, J.M. Glutathione in cancer cell death. Cancers 2011, 3, 1285–1310.
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-culf, M. Role of glutathione in cancer: From mechanisms to therapies. Biomolecules 2020, 10, 1–27.
- Wang, W.; Ben-Daniel, B.H.; Cohen, Y. Control of plant diseases by extracts of Inula viscosa. Phytopathology 2004, 94, 1042–1047.
- Widhalm, J.R.; Dudareva, N. A familiar ring to it: Biosynthesis of plant benzoic acids. Mol. Plant 2015, 8, 83–97.
