The widespread genus Cirsium Mill. (thistle) is one of the biggest genera in Asteraceae family (subfamily: Carduoideae Cass. Ex Sweet, tribe: Cardueae Cass., subtribe: Carduinae (Cass.) Dumort, sect. Cirsium). It includes about 250 species spread throughout Europe, North Africa, East Asia, Central Asia, SW Asia and North and Central America. Its species have been used for many years as a traditional herbal medicine. As the origin of the name suggests (“khirsos” in Greek means “swollen veins”), the genus Cirsium has been known for centuries for its usage against varicose diseases, to relieve pain.
- Cirsium appendiculatum
- UHPLC–HRMS
- biochemometric
- antioxidants
- enzyme inhibitory activity
- partial least-square discriminant analysis
1. Introduction
2. Results and Discussion

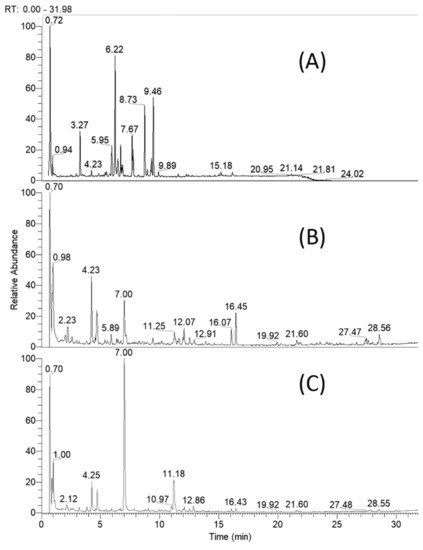
2.1. UHPLC–HRMS Profiling of Specialized Natural Products in Cirsium appendiculatum Extracts
| № | Identified/Tentatively Annotated Compound | Molecular Formula | Exact Mass [M-H]− |
tR (Min) |
Δ ppm | Distribution | Level of Identification (CAWG) |
|---|---|---|---|---|---|---|---|
| Carboxylic (including hydroxybenzoic and hydroxycinnamic) acids | |||||||
| 1. | protocatechuic acid a | C7H6O4 | 153.0179 | 2.15 | −7.986 | 1, 2 | 1 |
| 2. | dihydroxybenzoic acid | C7H6O4 | 153.0181 | 3.44 | −8.182 | 2, 3 | 2 |
| 3. | gentisic acid a | C7H6O4 | 153.0179 | 3.86 | −9.685 | 2 | 1 |
| 4. | vanillic acid a | C8H8O4 | 167.0338 | 4.77 | −6.837 | 1, 2, 3 | 1 |
| 5. | caffeic acid a | C9H8O4 | 179.0340 | 3.51 | −5.317 | 1, 2, 3 | 1 |
| 6. | quinic acid | C7H12O6 | 191.0551 | 3.18 | −5.032 | 1, 2, 3 | 2 |
| 7. | eucomic acid | C11H12O6 | 239.0557 | 3.38 | −0.717 | 1, 2, 3 | 2 |
| 8. | caffeoyl-syringic acid | C18H16O8 | 359.0985 | 2.32 | 0.390 | 1, 2, 3 | 4 |
| Hydroxybenzoic and hydroxycinnamc acids glycosides | |||||||
| 9. | 4-hydroxyphenylacetic acid O-β-D-glucoside |
C14H18O8 | 313.0933 | 2.18 | 1.467 | 2 | 2 |
| 10. | vanillic acid O-deoxyhexoside | C14H18O8 | 313.0934 | 3.25 | 1.467 | 2, 3 | 2 |
| 11. | gentisic acid O-hexoside | C14H20O8 | 315.1087 | 1.92 | 0.601 | 1, 2, 3 | 2 |
| 12. | p-hydroxybenzoic acid O-hexoside | C14H20O8 | 315.1086 | 2.10 | 0.029 | 1, 2, 3 | 2 |
| 13. | vanillic acid O-hexoside | C14H18O9 | 329.0885 | 1.71 | 2.020 | 1, 2, 3 | 2 |
| 14. | leonuriside A | C14H20O9 | 331.1037 | 1.44 | 0.739 | 1, 2, 3 | 2 |
| 15. | gallic acid O-hexoside | C13H16O10 | 331.0676 | 1.58 | 1.601 | 2 | 2 |
| Acylquinic acids | |||||||
| 16. | 1-p-coumaroylquinic acid | C16H18O8 | 337.0932 | 4.61 | 1.007 | 1, 2 | 2 |
| 17. | 3-p-coumaroylquinic acid | C16H18O8 | 337.0935 | 3.01 | 1.748 | 2 | 2 |
| 18. | 1-caffeoylquinic acid | C16H18O9 | 353.0880 | 2.27 | 0.410 | 1, 2 | 2 |
| 19. | neochlorogenic (3-caffeoylquinic) acid | C16H18O9 | 353.0878 | 3.21 | −0.015 | 1, 2, 3 | 1 |
| 20. | chlorogenic (5-caffeoylquinic) acid a | C16H18O9 | 353.0874 | 3.94 | −1.233 | 1, 2, 3 | 1 |
| 21. | 4-caffeoylquinic acid | C16H18O9 | 353.0879 | 6.27 | 0.155 | 1, 2, 3 | 2 |
| 22. | 3,4-dicaffeoylquinic acid a | C25H24O12 | 515.1199 | 5.73 | 0.836 | 1, 2, 3 | 1 |
| 23. | 1,5-dicaffeoylquinic acid a | C25H24O12 | 515.1191 | 5.91 | −0.697 | 1, 2, 3 | 1 |
| 24. | 3,5-dicaffeoylquinic acid | C25H24O12 | 515.1199 | 6.08 | 0.720 | 1, 2, 3 | 1 |
| 25. | 4,5-dicaffeoylquinic acid | C25H24O12 | 515.1191 | 6.25 | −0.697 | 1, 2, 3 | 1 |
| 26. | 1,3,5-tricaffeoylquinic acid | C34H30O15 | 677.1512 | 5.15 | - | 1, 2, 3 | 1 |
| Flavonoids | |||||||
| 27. | apigenin a | C15H9O5 | 269.0459 | 8.58 | 1.313 | 1, 3 | 1 |
| 28. | genkwanin a | C16H12O5 | 283.0608 | 11.41 | −1.543 | 2 | 1 |
| 29. | acacetin | C16H12O5 | 283.0615 | 11.40 | 1.142 | 1, 3 | 2 |
| 30. | luteolin a | C15H10O6 | 285.0404 | 7.55 | −0.075 | 1, 3 | 1 |
| 31. | hispidulin (scutellarein-6-methyl ether) a | C16H12O6 | 299.0561 | 8. 81 | −0.172 | 1, 2, 3 | 1 |
| 32. | diosmetin | C16H12O6 | 299.0560 | 9.28 | −0.272 | 1 | 1 |
| 33. | quercetin a | C15H9O6 | 301.0354 | 7.61 | 1.11 | 1 | 1 |
| 34. | pectolinarigenin | C17H14O6 | 313.0722 | 12.26 | 1.305 | 1, 2, 3 | 2 |
| 35. | nepetin (6-methoxyluteolin) | C16H11O7 | 315.0514 | 8.09 | 1.251 | 1, 3 | 2 |
| 36. | cirsiliol | C17H14O7 | 329.0669 | 8.87 | 0.772 | 1 | 2 |
| 37. | apigenin 7-O-glucoside a | C21H20O10 | 431.0988 | 6.06 | 0.835 | 1 | 1 |
| 38. | kaempferol 3-O-deoxyhexoside | C21H20O10 | 431.0983 | 6.60 | −0.232 | 1, 2 | 2 |
| 39. | apigenin O-hexuronide | C21H18O11 | 445.0770 | 6.45 | −0.347 | 1, 2, 3 | 2 |
| 40. | kaempferol 3-O-glucoside a | C21H20O11 | 447.0935 | 5.63 | 0.571 | 1, 2 | 1 |
| 41. | luteolin 7-O-glucoside a | C21H19O11 | 447.0934 | 6.04 | 0.281 | 1, 2, 3 | 1 |
| 42. | luteolin 7-O-hexuronide | C21H18O12 | 461.0734 | 5.37 | 1.911 | 1, 3 | 2 |
| 43. | diosmetin 7-O-hexoside | C22H22O11 | 461.1092 | 6.30 | 0.684 | 1, 2, 3 | 2 |
| 44. | hispidulin 7-O-hexoside | C22H22O11 | 461.1093 | 6.67 | 0.966 | 1, 2, 3 | 2 |
| 45. | hispidulin-O-hexuronide | C22H20O12 | 475.0882 | 6.33 | 0.002 | 1, 3 | 2 |
| 46. | pectolinarigenin-O-hexoside | C23H24O11 | 475.1247 | 8.11 | 0.159 | 1 | 2 |
| 47. | nepetin-O-hexoside | C22H21O12 | 477.1040 | 5.65 | 0.316 | 1, 3 | 2 |
| 48. | nepetin-O-hexuronide | C22H20O13 | 491.0835 | 6.32 | 0.725 | 1 | 2 |
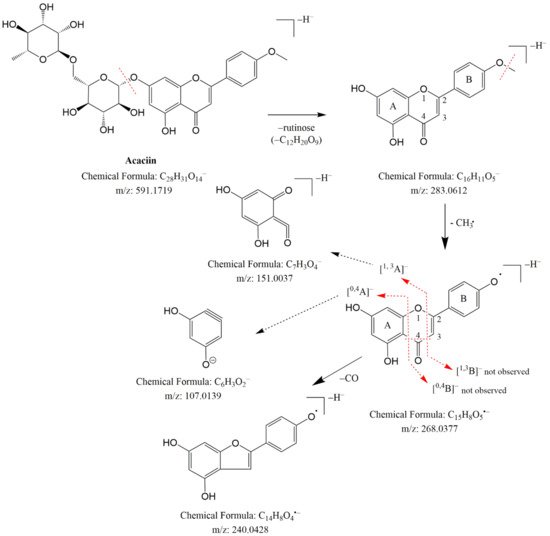
| 49. | acaciin (acacetin 7-O-rutinoside) a | C28H32O14 | 591.1730 | 7.59 | 3.622 | 1, 2, 3 | 1 |
| 50. | kaempferol 3-O-rutinoside a | C27H30O15 | 593.1532 | 5.40 | 3.383 | 1, 3 | 1 |
| 51. | hispidulin 7-O-rutinoside | C28H32O15 | 607.1675 | 6.34 | 1.049 | 1, 2, 3 | 2 |
| 52. | pectolinarin (pectolinarigenin 7-O- rutinoside) a |
C29H34O15 | 621.1824 | 7.67 | −0.199 | 1, 2, 3 | 1 |
| Free fatty acids | |||||||
| 53. | nonanedioic acid (azelaic acid) | C9H16O4 | 187.0967 | 6.32 | −4.502 | 1, 2, 3 | 2 |
| 54. | 3-hydroxysuberic acid | C8H14O5 | 189.0758 | 4.64 | −5.483 | 1, 2, 3 | 2 |
| 55. | 3-hydroxyazelaic acid | C9H16O5 | 203.0918 | 6.25 | −3.677 | 1, 2, 3 | 2 |
| 56. | 2-dodecenoic acid | C12H20O4 | 227.1287 | 9.46 | −0.715 | 2, 3 | 2 |
| 57. | 9,13-dyhidroxyoctadeca-9,11,13-trienoic acid | C18H30O4 | 309.2074 | 12.76 | 0.768 | 2, 3 | 2 |
| 58. | 11,12-dyhidroxyoctadeca-9,13,15-trienoic acid | C18H30O4 | 309.2075 | 12.91 | −0.332 | 2 | 2 |
| 59. | 9,10-dyhidroxyoctadeca-12,14,16-trienoic acid | C18H30O4 | 309.2074 | 10.81 | 1.835 | 2 | 2 |
| 60. | 9,13-dyhidroxyoctadeca-11,13-dienoic acid | C18H32O4 | 311.2231 | 13.67 | 0.859 | 1, 2, 3 | 2 |
| 61. | 9,10-dyhidroxyoctadeca-9-enoic acid | C18H34O4 | 313.2388 | 13.79 | 0.885 | 3 | 2 |
2.1.1. Carboxylic (Including Hydroxybenzoic, Hydroxycinnamic and Acylquinic) Acids and Their Glycosides
2.1.2. Flavonoids, Flavones and Flavonols
2.1.3. Methoxylated Flavonoids
2.2. Total Content of Phenolics and Flavonoids
| Parts | Total Phenolic Content (mgGAE/g) | Total Flavonoid Content (mgRE/g) | DPPH• (mgTE/g) | ABTS•+ (mgTE/g) | CUPRAC (mgTE/g) | FRAP (mg TE/g) |
PHMD (mmolTE/g) | Metal Chelating (mgEDTAE/g) |
|---|---|---|---|---|---|---|---|---|
| Flower heads | 71.75 ± 1.47 b | 46.59 ± 0.89 a | 101.79 ± 0.15 a | 224.57 ± 0.57 a | 356.97 ± 11.52 b | 169.60 ± 0.84 b | 1.71 ± 0.07 b | 32.53 ± 3.51 a |
| Aerial parts | 26.02 ± 1.49 c | 2.64 ± 0.08 c | 70.25 ± 1.91 c | 124.16 ± 4.73 b | 103.77 ± 5.89 c | 69.98 ± 2.01 c | 0.74 ± 0.01 c | 9.42 ± 0.54 b |
| Roots | 143.62 ± 2.99 a | 3.99 ± 0.06 b | 97.95 ± 0.60 b | 224.59 ± 0.33 a | 618.36 ± 5.17 a | 269.89 ± 8.50 a | 3.36 ± 0.15 a | na |
2.3. Antioxidant Properties
2.4. Enzyme Inhibitory Effects
| Parts | AChE Inhibition (mgGALAE/g) | BChE Inhibition (mgGALAE/g) | Tyrosinase (mgKAE/g) | Amylase (mmolACAE/g) | Glucosidase (mmolACAE/g) |
|---|---|---|---|---|---|
| Flower heads | 4.40 ± 0.40 a | 1.54 ± 0.07 c | 97.78 ± 0.76 c | 0.60 ± 0.01 a | 0.41 ± 0.10 b |
| Aerial parts | 3.52 ± 0.31 b | 2.67 ± 0.34 b | 110.61 ± 0.79 b | 0.61 ± 0.06 a | na |
| Roots | 4.93 ± 0.25 a | 3.80 ± 0.26 a | 127.99 ± 0.68 a | 0.62 ± 0.04 a | 0.72 ± 0.07 a |
This entry is adapted from the peer-reviewed paper 10.3390/plants10102046
References
- Wolfender, J.-L.; Litaudon, M.; Touboul, D.; Queiroz, E.F. Innovative omics-based approaches for prioritisation and targeted isolation of natural products–new strategies for drug discovery. Nat. Prod. Rep. 2019, 36, 855–868.
- Gevrenova, R.; Zheleva-Dimitrova, D.; Balabanova, V.; Voynikov, Y.; Sinan, K.I.; Mahomoodally, M.F.; Zengin, G. Integrated phytochemistry, bio-functional potential and multivariate analysis of Tanacetum macrophyllum (Waldst. & Kit.) Sch. Bip. and Telekia speciosa (Schreb.) Baumg.(Asteraceae). Ind. Crop. Prod. 2020, 155, 112817.
- Kadereit, J.W.; Jeffrey, C. Flowering Plants, Eudicots: Asterales. The Families and Genera of Vascular Plants, Volume VIII; Springer: Berlin/Heidelberg, Germany, 2007.
- Yildiz, B.; Arabaci, T.; Dirmenci, T.; Köstekci, S. A taxonomic revision of the genus Cirsium Mill. sect. Cirsium (Asteraceae: Cardueae) in Turkey. Turk. J. Bot. 2016, 40, 514–530.
- Kim, M.S.; Nam, M.; Hwang, G.S. Metabolic alterations in two Cirsium species identified at distinct phenological stages using UPLC-QTOF/MS. Phytochem. Anal. 2018, 29, 77–86.
- Lee, S.; Lee, D.-H.; Kim, J.-C.; Um, B.H.; Sung, S.H.; Jeong, L.S.; Kim, Y.K.; Kim, S.-N. Pectolinarigenin, an aglycone of pectolinarin, has more potent inhibitory activities on melanogenesis than pectolinarin. Biochem. Biophys. Res. Commun. 2017, 493, 765–772.
- Abbet, C.; Slacanin, I.; Corradi, E.; De Mieri, M.; Hamburger, M.; Potterat, O. Comprehensive analysis of Cirsium spinosissimum Scop., a wild alpine food plant. Food Chem. 2014, 160, 165–170.
- Jordon-Thaden, I.E.; Louda, S.M. Chemistry of Cirsium and Carduus: A role in ecological risk assessment for biological control of weeds? Biochem. Syst. Ecol. 2003, 31, 1353–1396.
- Nazaruk, J.; Chłędzik, S.; Strawa, J.; Bazydło, K.; Wajs-Bonikowska, A. Chemical Composition and Antioxidant Activity of Cirsium vulgare Inflorescences. Nat. Prod. Commun. 2017, 12, 1934578X1701200414.
- Do, J.-C.; Jung, K.-Y.; Son, K.-H. Isolation of pectolinarin from the aerial parts of Cirsium nipponicum. Korean J. Pharmacogn. 1994, 25, 73–75.
- Dutta, C.; Ray, L.P.; Roy, D. Taraxasterol and its derivatives from Cirsium arvense. Phytochemistry 1972, 11, 2267–2269.
- N. Stoyanov Stefanov, B.K. Flora Republicae Bulgaricae; Nauka i Izkustvo (Science and Art State Publishing House): Sofia, Bulgaria, 1967; Volume 2, pp. 1123–1128.
- Wolfender, J.L.; Queiroz, E.F.; Allard, P.M. Massive metabolite profiling of natural extracts for a rational prioritization of bioactive natural products: A paradigm shift in pharmacognosy. Food Front. 2020, 1, 105–106.
- Termentzi, A.; Zervou, M.; Kokkalou, E. Isolation and structure elucidation of novel phenolic constituents from Sorbus domestica fruits. Food Chem. 2009, 116, 371–381.
- Chahdoura, H.; João, C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.; Achour, L. Phytochemical characterization and antioxidant activity of Opuntia microdasys (Lehm.) Pfeiff flowers in different stages of maturity. J. Funct. Foods 2014, 9, 27–37.
- Ak, G.; Gevrenova, R.; Sinan, K.I.; Zengin, G.; Zheleva, D.; Mahomoodally, M.F.; Senkardes, I.; Brunetti, L.; Leone, S.; Di Simone, S.C. Tanacetum vulgare L.(Tansy) as an effective bioresource with promising pharmacological effects from natural arsenal. Food Chem. Toxicol. 2021, 153, 112268.
- Gai, X.-H. Research progress on chemical constituents of Coptidis Rhizoma and its pharmacological activities. Chin. Tradit. Herb. Drugs 2018, 4919–4927.
- Münzenberger, B.; Heilemann, J.; Strack, D.; Kottke, I.; Oberwinkler, F. Phenolics of mycorrhizas and non-mycorrhizal roots of Norway spruce. Planta 1990, 182, 142–148.
- Lee, S.R.; Clardy, J.; Senger, D.R.; Cao, S.; Kim, K.H. Iridoid and phenylethanoid glycosides from the aerial part of Barleria lupulina. Rev. Bras. Farmacogn. 2016, 26, 281–284.
- Puppala, M.; Ponder, J.; Suryanarayana, P.; Reddy, G.B.; Petrash, J.M.; LaBarbera, D.V. The isolation and characterization of β-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis. PLoS ONE 2012, 7, e31399.
- Clifford, M.N.; Wu, W.; Kirkpatrick, J.; Kuhnert, N. Profiling the chlorogenic acids and other caffeic acid derivatives of herbal Chrysanthemum by LC− MS n. J. Agric. Food Chem. 2007, 55, 929–936.
- Jaiswal, R.; Kiprotich, J.; Kuhnert, N. Determination of the hydroxycinnamate profile of 12 members of the Asteraceae family. Phytochemistry 2011, 72, 781–790.
- Justesen, U. Collision-induced fragmentation of deprotonated methoxylated flavonoids, obtained by electrospray ionization mass spectrometry. J. Mass Spectrom. 2001, 36, 169–178.
- Ren, D.; Ran, L.; Yang, C.; Xu, M.; Yi, L. Integrated strategy for identifying minor components in complex samples combining mass defect, diagnostic ions and neutral loss information based on ultra-performance liquid chromatography-high resolution mass spectrometry platform: Folium Artemisiae Argyi as a case study. J. Chromatogr. 2018, 1550, 35–44.
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298.
- Nazaruk, J. Antioxidant activity and total phenolic content in Cirsium five species from north–east region of Poland. Fitoterapia 2008, 79, 194–196.
- Llorent-Martínez, E.J.; Zengin, G.; Sinan, K.I.; Polat, R.; Canlı, D.; Picot-Allain, M.C.N.; Mahomoodally, M.F. Impact of different extraction solvents and techniques on the biological activities of Cirsium yildizianum (Asteraceae: Cynareae). Ind. Crop. Prod. 2020, 144, 112033.
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999.
- Wu, L.; Wu, W.; Cai, Y.; Li, C.; Wang, L. HPLC fingerprinting-based multivariate analysis of phenolic compounds in mango leaves varieties: Correlation to their antioxidant activity and in silico α-glucoidase inhibitory ability. J. Pharm. Biomed. Anal. 2020, 191, 113616.
- Ramsay, R.R.; Tipton, K.F. Assessment of enzyme inhibition: A review with examples from the development of monoamine oxidase and cholinesterase inhibitory drugs. Molecules 2017, 22, 1192.
- Tan, Y.; Chang, S.K. Digestive enzyme inhibition activity of the phenolic substances in selected fruits, vegetables and tea as compared to black legumes. J. Funct. Foods 2017, 38, 644–655.
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2020, 338, 128119.
- Yener, I.; Kocakaya, S.O.; Ertas, A.; Erhan, B.; Kaplaner, E.; Oral, E.V.; Yilmaz-Ozden, T.; Yilmaz, M.A.; Ozturk, M.; Kolak, U. Selective in vitro and in silico enzymes inhibitory activities of phenolic acids and flavonoids of food plants: Relations with oxidative stress. Food Chem. 2020, 327, 127045.
- Liao, Z.; Chen, X.; Wu, M. Antidiabetic effect of flavones from Cirsium japonicum DC in diabetic rats. Arch. Pharmacal Res. 2010, 33, 353–362.
