2. Methamphetamine
Methamphetamine (also pervitin, methylamphetamine, desoxyephedrine, and methedrine) belongs to the group of wake-promoting amines [
14]. The first discovered member of this group, amphetamine, was synthesized in 1887. The group of wake-promoting amines includes hundreds of substances, which were (and still are) often used as a treatment for exhaustion, narcolepsy, increased appetite, and sometimes abused by the military in order to increase the performance of combat units. The base substance for MA synthesis is ephedrine, along with lye and red phosphorus used in its manufacture. A minimum level of knowledge of at least high school grade is generally required, since when done imperfectly this may threaten the health of the user [
9]. Pure MA can be obtained in crystalline form; it is, however, more common to be sold as a microcrystalline white powder with a bitter taste and without a perceptible aroma. Due to the presence of additives originating from home manufacturing, the powder often has a yellow or purple discoloration [
15].
MA can be classified as a non-catecholamine sympathomimetic substance, showing a sympathomimetic effect even without possessing the catecholamine structure [
14]. MA exhibits stimulatory effects on the CNS [
16,
17,
18] by releasing noradrenaline (NA) from noradrenergic neurons that act on alpha receptors [
19]. Amphetamines, on the other hand, have little influence on these receptors. Both serotonin (5-hydroxytryptamine; 5-HT) and dopamine are also released after the administration of MA, leading to increased psychomotor activity. MA initially induces alertness, suppresses fatigue, and increases energy; with higher doses, it produces feelings of euphoria, blissfulness, and increased self-confidence [
20,
21].
MA accelerates heart activity and increases blood pressure, which leads to hyperthermia, bronchial dilation, and pupil dilation [
22]. A strong psychological addiction is created with MA, but without physical addiction [
23]. MA is well absorbed by the gastrointestinal tract and mucous membranes. Its lipophilic character enables it to pass through the blood–brain barrier with greater ease compared to amphetamine [
24]. Once in the CNS, MA causes an increase in monoamine mediator (dopamine, noradrenaline, serotonin) concentrations in the synapses and in the cytosols of neurons. Simultaneously, neurotransmitter reabsorption is inhibited and the degradation of MA is reduced [
25]. In high doses, monoamine oxidase (MAO) is also inhibited. Drug inactivation via MAO is prevented due to the presence of a methyl group on the alpha carbon [
24]. Benefiting from this protection, MA is widely distributed throughout the body, and its biological half-life is 12 to 34 h [
26].
After the effects subside, there is a lack of neuromodulators, leading to an unpleasant state, which is often called withdrawal syndrome. During long-term use, irreversible changes appear in the mitochondrial metabolism, potentially leading to apoptosis of the damaged neuron [
25]. Sexual activity is also affected by long-term use. Other effects include increased nervousness, restlessness, insomnia, bruxism, reduced appetite, and subsequent weight loss [
27]. Restlessness and paranoia may potentially lead to paranoid psychosis, which can recur even after discontinuation of drug use [
28].
Research has determined that different animal species metabolize MA differently. Rats, for example, use aromatic hydroxylation during its metabolism, whereas rabbits use deamination [
24,
29]. In humans, a substantial amount of MA is excreted via the urine in an unaltered form (approximately 45% within 24 h). Excretion is highly dependent on pH; while up to 76% can be excreted in acidic urine, only about 2% can be excreted in alkaline urine [
30,
31]. MA metabolites are also excreted in urine—approximately 15% is metabolized by hydroxylation in the liver to hydroxymethamphetamine. About 7% is metabolized by N-demethylation to amphetamine, which is further processed to hydroxyamphetamine (2–4%) and norephedrine (2%), and subsequently hydroxynorephedrine (0.3%) and phenylacetone (0.9%), which are metabolized to benzoic acid and hippuric acid, respectively [
24,
29].
2. Influence of Methamphetamine on the CNS and on Selected Parts of the Brain
Every living organism strives for a dynamic balance, called homeostasis. While under the effect of addiction, this balance is threatened by distinct physical and cognitive events, leading to increased stress in the addicted individual [
32,
33]. Consequently, their behavior is changed; unless the event corresponds to a cognitive depiction based on previous subjective experience, it is accompanied by an increase in excitement, alertness, and cognitive processing [
34,
35,
36,
37,
38,
39]. The interface between the incoming sensory input and the evaluation process consists of limbic brain structures, including the hippocampus and prefrontal cortex, and the structures directly influencing the limbic system, i.e., the striatum [
40].
The control areas in the brain send signals that configure the processing of incoming sensory inputs from moment-to-moment [
37,
41]. Humans possess unique cognitive flexibility. The brain control system consists of functionally diverse areas that are anatomically separated from the remaining systems of neural response processing [
41].The ability to perform countless tasks requires control functions that persist in time and that are capable of not only preventing attention diversion, but of responding quickly to unpredictable demands as well [
33].
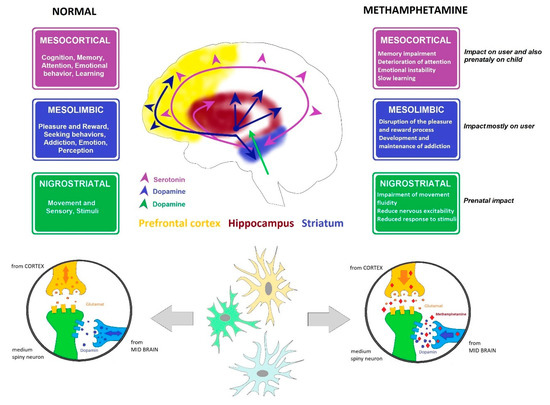
The selection of samples from rat brains was based on the fact that MA interferes mostly with dopaminergic and serotonergic neural response pathways. MA enters the terminals or neuron via the monoamine transporters (dopamine transporter, serotonin transporter, or norepinephrine transporter), displaces both vesicular and intracellular monoamines, and facilitates the release of monoamines into the extraneuronal space by synaptic transport in the monoamine transporters. Both pathways (dopaminergic and serotoninergic) connect parts of the brain responsible for motor control, fear, pleasure, reward, and addiction mechanisms (
Figure 2) [
22,
42].
Figure 2. Dopamine and serotonin pathways. Cross-section through the brain showing dopamine and serotonin pathways and their main functions, including affection, mood, memory, sleep, pleasure, reward, and compulsive behavior, and the influence of MA.
2.1. Dopaminergic System
Dopamine is a neurohormone; its release from the hypothalamus inhibits prolactin secretion from adenohypophysis, affects motor system control in the CNS, initiates various behavioral pattern, and modulates the activity of visceral functions. Dopamine is a low molecular weight neurotransmitter and is categorized as a catecholamine. In the bloodstream, dopamine exhibits sympathomimetic effects, such as increasing the systolic blood pressure and the heart rate [
43,
44]. Dopamine is synthesized from tyrosine or phenylalanine and serves as a precursor for both adrenaline and NA; MAO and catechol-O-methyltransferase (COMT) can degrade it, with homovanillic acid being the final product [
45].
Dopaminergic nuclei in the brain are designated as A8, A9, and A10. The most significant are located in the substantia nigra pars compacta (A9) and medially in the ventral tegmental area (A10). Fibers from the substantia nigra are projected into the striatum, and to a lesser extent into the globus pallidus. Fibers from the ventral tegmental area create the mesolimbic dopaminergic system, ending in the ventral striatum, ventral pallidum, septum verum, the amygdala, and the cerebral cortex, mainly the prefrontal cortex and primary motor cortex (Figure 2).
Reduced dopamine concentrations in the striatum causes hypokinesis and muscle tremors; lowering the concentration in the prefrontal cortex leads to memory, attention, and motivation disorders. Using MA increases the amount of dopamine accessible to D3 receptors [
46], directly stimulating the reward center in the brain. Repeated administration of MA induces long-lasting deficits in the striatal concentrations of dopamine and its metabolites, tyrosine hydroxylase activity, and dopamine transporter binding sites [
17,
46].
MA causes degeneration of striatal nerve terminals, as shown by long-lasting depletion of dopamine concentration, dopamine transporter, and vesicular monoamine transporter 2 levels. The ability of MA to mobilize dopamine from intraneuronal stores to the extracellular space via dopamine-transporter-mediated outward transport results in elevated extracellular dopamine concentrations. The neurotoxic effects of MA are postulated to occur from subsequent auto-oxidation of dopamine to highly reactive free radicals [
17,
22,
46]. An alternative model suggests that redistribution of dopamine from vesicular storage pools to the cytoplasmic compartment, allowing intraneuronal oxidation, is the primary cause of dopamine terminal injury [
46].
The principle reason lies in the stimulation of the tyrosine kinase receptor (e.g., insulin-like growth factor 1 receptor (IGF-1R)). It is shown to mediate activation of the phosphatidylinositol-3 kinase (PI3K)/Akt signaling pathway, an important mediator of cell survival. PI3K is activated by estrogen, the G-protein-coupled estrogen receptor 1 (GPER1, also known as GPR30). Akt controls expression of the anti-apoptotic molecule Bcl-2 via the cAMP-response element-binding protein (CREB), and can also phosphorylate glycogen synthase kinase 3β (GSK3β) and BAD proteins, thereby inhibiting their pro-apoptotic functions [
17,
22].
This suggests that the balance among vesicular, cytoplasmic, and extracellular dopamine pools play a key role in the neurotoxic action of MA.
Long-term use leads to a loss of dopaminergic receptors and to reduced dopamine production in general, causing withdrawal states in addicted individuals and triggering the need to increase the dosage of the abused substance [
47,
48] (
Figure 2).
2.2. Serotoninergic System
The serotoninergic system in the CNS has a regulatory effect on many functions and modulates the activity of other projection systems. This system is closely linked to the noradrenergic system, which it often supplements. Serotonin is derived from L-tryptophan as 5-HT. Serotonin inactivation is catalyzed through two enzymes, MAO and aldehyde dehydrogenase; the activity of these enzymes leads to the creation of 5–hydroxyindoleacetate (5-hydroxyindoleacetic acid), which is mostly excreted in the urine (as a glucuronic acid conjugate) [
49]. Another serotonin metabolic pathway results in the synthesis of melatonin; initially, an acetyl group binds to the amino group of serotonin, resulting in N-acetylserotonin. Subsequently, a methyl group binds the hydroxyl group, creating melatonin.
There are seven known subtypes of serotoninergic receptors (i.e., 5-HT1-7R), both excitatory and inhibitory. The effect of serotonin is strongly dependent on the receptors expressed on the neurons of any given structure. The soma of most neurons in this system are in the raphe nuclei of the reticular formation. Their axons enter both ascending and descending columns, reaching all cortical areas (including the prefrontal cortex) and all limbic system structures (including the hippocampus), with others reaching the striatum, thalamus, hypothalamus, brain stem, cerebellum, and spinal cord. Excessive activity in ascending columns leads to mood changes and behavior disorders. The axons passing through the dorsal horn of the spinal cord are associated with pain transmission (Figure 2).
Reductions in forebrain concentrations of serotonin (5-HT) and its metabolites and a decrease of tryptophan hydroxylase activity after MA administration have been described. Lowered serotonin synthesis causes depression and sleep disorders [
17,
22,
50]. MA also causes hyperthermia, which can be lethal. Release of monoamines into the extraneuronal space by synaptic transport in the monoamine transporters suggests that dopamine receptor activation is crucial for methamphetamine-induced hyperthermia. However, when dopamine pools are empty, serotonin starts to play the main role. MA exerts a hyperthermic effect via dopamine transporters, or via serotonin transporters if the dopamine transporters are absent [
22].
2.3. The Striatum
The striatum (also called the corpus striatum) is grey matter deep in the telencephalon. It is considered the most significant part of the basal ganglia (evolutionarily one of the oldest structures). The basal ganglia participate in generating and controlling movement, cognitive functions, and the functions of the limbic system.
The basal ganglia are connected to various cerebral pathways; the general signal pathway leads from the cerebral cortex to the basal ganglia. The signal continues from the basal ganglia to the thalamus and finally back to the cerebral cortex. Four types of basal ganglia loops have been described: motor loops, oculomotor loops, limbic circuits, and associative pathways. While describing them, it is important to determine the part of the cerebral cortex from which the stimuli originate; furthermore, it is equally necessary to ascertain the ganglia involved, i.e., functioning as inputs or outputs, and the specific functional area of the cortex that is the intended destination. The information comes predominantly from the motor and somatosensorial areas of the cortex and enters the putamen, taking either direct or indirect pathways. The function of the direct pathway is to support the movement, while the indirect pathway inhibits the thalamus, which increases the excitatory influence on the cortex. Increasing the activity in the direct pathway leads to increased motor activity, while the indirect pathway serves mainly to suppress unwanted movements. The activity in both pathways should be balanced; disturbing this balance leads to hyperkinetic and hypokinetic disorders, respectively [
32,
51].
The substantia nigra pars compacta, which is rich in dopaminergic neurons, plays a substantial role in the modulation of pathway activity. Dopamine increases the activity of the direct pathway via D1 receptors, while also decreasing indirect pathway activity via D2 receptors ([
32,
45,
52]. The basal ganglia participate in motor function control (and to some extent, cognitive function control as well). Generally, they exert an inhibitory influence on motor activity; motor cortex neurons suppress cortical stimuli both through direct feedback and through the reticular formation. The neurons are activated even before the movement begins, possibly indicating that they take part in its planning as well [
52]. It is generally assumed that they also participate in the control mechanisms of intricate movement patterns, such as writing, ball games, and speech [
53].
2.4. The Prefrontal Cortex
The prefrontal cortex represents one of the largest cortical areas of the human brain, accounting for roughly 29% of its volume, and is responsible for higher functions. The cortex area of the frontal lobe, which does not belong to motor areas, represents a significant part of the association cortex [
54]. There are links to the visual, olfactory, and auditory cortices, providing integration of sensory inputs. The prefrontal cortex, which receives information from various sources, is responsible for planning, decision-making, and creating new ideas. It has connections to the entire brain, especially to the rostral thalamus.
It is the only area of the cortex with projections directly into the hypothalamus and septal areas, with a significant role in the regulation of the limbic system. There is a direct bidirectional link between them, with the prefrontal cortex taking part in learning and memory processes [
54,
55]. Damage to the prefrontal cortex manifests in severe psychological disorders, including apathy, memory loss, aggression, obsession, loss of social restraint, and emotional instability [
54,
56,
57,
58,
59].
2.5. Hippocampus
The hippocampus is a paired structure in the telencephalon, occupying the middle part of the temporal lobe in both hemispheres. Information from the cerebral cortex and limbic system is processed in the hippocampus and subsequently sent to the frontal thalamus, then ultimately back to the cerebral cortex. This rhythmic, repetitive activity of the hippocampus creates a basis for complex integration processes, such as storing of information as long-term memory [
60]. It is part of the limbic system, mediating both short-term and long-term memory and orientation in space [
61,
62,
63]. The ability to orientate in space and time allows the individual to perceive their position in relation to their environment, as well as in relation to changes in velocity and positioning in time [
61,
64]. Any damage can manifest as learning disorders (long-term memory), disruptions to short-term memory, and even complete amnesia [
65].
There is a large number of cells encoding the perception of space in the brain; they are located both in the hippocampus and in other structures, such as the cerebellum and cerebral cortex [
66]. The structure of the hippocampus is essentially identical in most mammals [
41,
67,
68,
69]. If the subject is placed in a confined and defined space, the pyramidal cells in this area of the brain (along with the granule cells in the cerebellum) begin to produce strong excitatory signals [
70,
71,
72,
73]. An analysis of the involved cells can be performed using the open arena experiment with free movement or using either the radial or Morris water mazes [
74,
75]. The cells are less sensitive to the vertical dimension in land-based mammals (e.g., rats), while in bats all three basic dimensions produce strong signals [
61,
76]. The cells associated with head orientation, found in several brain structures linked to the hippocampus, were discovered by Taube et al. [
77]. These cells produce signals when the animal looks in a certain direction; unlike the visual angle, the position in space is not relevant for the function of these cells.
Additionally, cells sensitive to both the head direction and general orientation of the animal in space were also found in the hippocampus [
61,
76,
78]. Any disruption of this structure can lead to the emotional instability of the animal, manifesting as anxiety states, depression, or obsession [
56,
79,
80,
81,
82].