Children show important developmental and maturational changes, which may contribute greatly to pharmacokinetic (PK) variability observed in pediatric patients. These PK alterations are further enhanced by disease-related, non-maturational factors. Specific to the intensive care setting, such factors include critical illness, inflammatory status, augmented renal clearance (ARC), as well as therapeutic interventions (e.g., extracorporeal organ support systems or whole body hypothermia [WBH]). This entry illustrates the relevance of both maturational and non-maturational changes in absorption, distribution, metabolism and excretion (ADME) applied to antibiotics.
- pediatric
- antibiotic
- critical illness
- pharmacokinetics
- augmented renal clearance
- extracorporeal membrane oxygenation
- continuous renal replacement therapy
- cardiopulmonary bypass
- whole body hypothermia
- therapeutic drug monitoring
1. Introduction
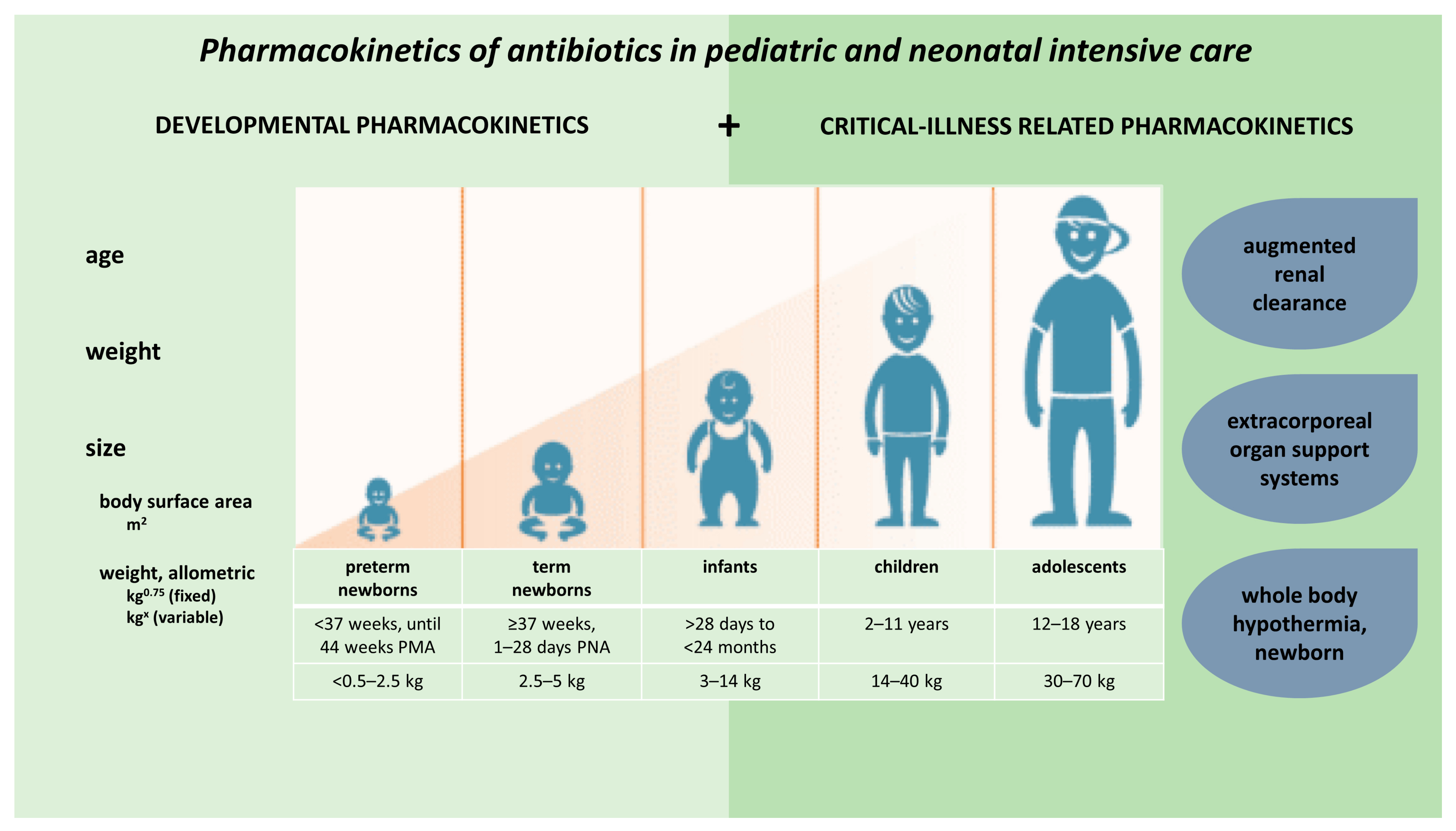
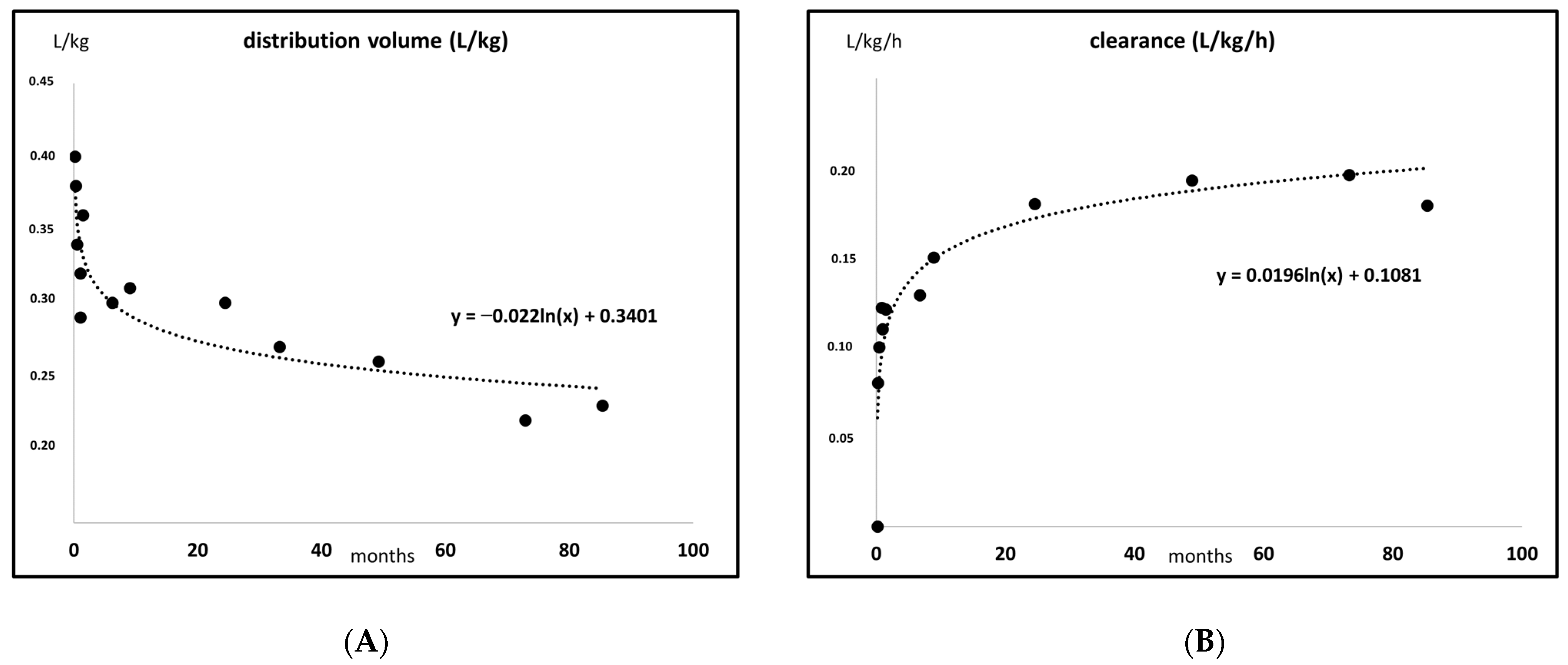
2. Developmental Pharmacokinetics, Applied to Antibiotics
|
Age |
Total Body Water/Weight % |
Extracellular Water/Weight % |
Intracellular Water/Weight % |
Plasma Albumin g/L |
Plasma Total Protein d/L |
|---|---|---|---|---|---|
|
Preterm, 2 kg |
82 |
44 |
34 |
26 |
40 |
|
Term, at birth |
78 |
40 |
32 |
28 |
43 |
|
7–30 days |
74 |
32 |
43 |
29 |
53 |
|
1–3 months |
73 |
30 |
42 |
29 |
54 |
|
3–6 months |
70 |
30 |
42 |
29 |
54 |
|
6–12 months |
60 |
27 |
35 |
29 |
54 |
|
1–3 year |
58–63 |
27–21 |
38–34 |
31 |
59 |
|
>3–6 year |
62 |
21 |
46 |
31 |
62 |
|
>6–18 year |
61–57 |
22–18 |
43–42 |
32 |
59 |
|
Adult |
59 |
19 |
40 |
40 |
63 |
3. Critical Illness Related Pharmacokinetic Alterations in Children and Infants
|
Category Based on the In Vitro Pattern of Antibacterial Activity |
Antibiotic Class |
PK/PD Target for Efficacy |
|---|---|---|
|
Time-dependent with minimal-to-no persistent effect |
Beta-lactams, Lincosamides |
% fT>MIC |
|
Time-dependent with moderate-to-persistent effect |
Glycopeptides, Oxazolidinones, Macrolides, Tetracyclines, Glycylcyclines, Polymyxins |
AUC0–24/MIC % fT>MIC (erythromycin, linezolid) |
|
Concentration-dependent with moderate-to-persistent effect |
Aminoglycosides, Fluoroquinolones, Lipopeptides, Metronidazole, Ketolides |
AUC0–24/MIC Cmax/MIC |
|
Antibiotic |
Number of Studies |
Study Aspects |
Age and Weight Range |
Results and Clinical Implications |
|---|---|---|---|---|
|
Vancomycin |
19 |
4 prospective studies (n = 168) [50][51][52][53] 10 retrospective TDM studies (n = 1120) [54][55][56][57][58][59][60][61][62][63] 5 retrospective popPK studies (n = 704) [64][65][66][67][68] |
0–18 y 0.68–108 kg |
Conflicting results with regard to association between Cmin and acute kidney injury [54][55][57][62] Eight studies reported CL and Vd. Mean Vd 0.44–1.04 L/kg. Mean CL 0.072–0.19 L/kg/h [51][52][53][56][64][65][67][68] Eight studies reported measured, simulated or estimated AUCs [50][51][52][56][62][66][67][68] Only two studies used continuous dosing [50][66] Substantial percentage of target non-attainment with standard dosing regimens (up to 92%, mostly subtherapeutic but also supratherapeutic concentrations) [50][51][52][54][55][56][59][64][66][67][68] Dosing of 60 mg/kg/day q8h advised if no renal impairment [61][67] One study advised lower doses for neonates (30 mg/kg/day), infants (35–40 mg/kg/day) and children (45 mg/kg/day) [59] Another study in neonates and infants <2m advised 14–18 mg/kg q8–12 h [68] |
|
Teicoplanin |
4 |
2 prospective cohort studies (n = 33) [69][70] 1 RCT (n = 20) [71] 1 prospective popPK study with dosing simulations (n = 42) [72] |
7 d–15.6 y 3.74–56 kg |
Three studies found that higher than standard dosing is needed to achieve Target attainment [70][71][72] One study found lower target attainment in older children (>1 y) compared to younger infants (<1 y) due to larger Vd and higher CL [71] Routine TDM of unbound concentrations was recommended due to highly variable unbound concentrations [72] |
|
Gentamicin |
4 |
1 retrospective TDM study (n = 140) [73] 1 prospective popPK study with dosing simulations (n = 36) [74] 2 studies investigating application of a Bayesian forecasting program (n = 117) [75][76] |
0 d–15 y Body weight not reported |
Higher initial doses and/or extended dosing interval in neonates and (young) infants [74][75] Two studies found age and weight to be significant predictors for Vd and/or CL [73][74] One study also found serum creatinine to be a significant predictor for the elimination constant (k) [73] |
|
Amikacin |
3 |
1 RCT (n = 60) [77] |
6 m–17 y 8–90 kg |
Higher doses per kg needed for neonates and infants (<1 y) due to higher Vd and CL [77] Higher Vd and CL in burn patients [79] |
|
Netilmicin |
1 |
1 prospective study (n = 9) [80] |
1 m–15.5 y 3.4–70 kg |
Mainly neonates Once daily 6 mg/kg is sufficient |
|
Piperacillin/tazobactam |
5 |
4 popPK studies with dosing simulations (n = 139) [81][82][83][84] 1 prospective study (n = 14) [46] |
0.1–18 y 2.7–53 kg |
High median eGFRSchwartz in all studies (lowest median eGFRSchwartz 142 mL/min/1.73 m2) [83] Median Vd: 0.24–0.444 L/kg (highest in neonates); Median CL: 0.19–0.299 L/kg/h [81][82][83][84] Insufficient target attainment with standard dosing. Extended infusion over >1 h needed for >90% probability of target attainment [46][81][82][83][84] |
|
Cefotaxime |
3 |
2 prospective studies (n = 39) [85][86] 1 prospective popPK study with dosing simulations (n = 49) [87] |
0–19 y 2.5–70 kg |
Neonates had longer elimination half-life [87] Continuous infusion needed for optimal target attainment and/or less susceptible microorganisms [85][87] |
|
Cefuroxime |
1 |
1 prospective cohort study (n = 11) [88] |
4 m–14 y 5.1 kg–45 kg |
Vd and CL higher in children with mechanical ventilation vs. children without mechanical ventilation and controls The elimination half-life is longer in critically ill children vs. controls |
|
Ceftriaxone |
1 |
Prospective popPK study with dosing simulations (n = 45) [89] |
0.1–16.7y |
Vd and CL comparable to non-critically ill children aged 1–6 y Vd and CL higher than non-critically ill children with cystic fibrosis 100 mg/kg q24h sufficient for most critically ill children and neonates 50 mg/kg q12h if eGFRSchwartz > 80 mL/min/1.73 m2 or increased MIC ≥0.5 mg/L |
|
Ceftolozane/tazobactam |
1 |
1 case series (n = 3) [90] |
8–19 m 5.8–11 kg |
Normal renal function: 35 mg/kg q8h appropriate for multidrug-resistent Pseudomonas aeruginosa Acute kidney injury: reduced dose 10 mg/kg q8h appropriate |
|
Ceftaroline |
1 |
1 prospective study (n = 7) [91] |
1–13 y 12.6–40.1 kg |
Higher median CL and Vd than reported in the package insert (non-critically ill children) Higher dosing and shorter dosing interval than package insert needed (15 mg/kg q6h) |
|
Amoxicillin/clavulanic acid |
3 |
1 prospective study (n = 15) [92] 1 prospective popPK study with dosing simulations (n = 50) [93] 1 meta-analytical modelling study (n = 44) [94] |
1 d–15 y 1.7–65 kg |
Higher amoxicillin CL than critically ill adults, comparable amoxicillin Vd and clavulanic acid CL and Vd [92][93] 25 mg/kg q4h as bolus or 1h infusion, depending on renal function, needed for optimal target attainment [93] Meta-modelling study (in neonates and young infants (<60 d) [94]: Sepsis is associated with lower amoxicillin concentrations and longer elimination half-life. Fixed dosing regimen: 125 mg and 250 mg q12h depending on body weight <4 kg or ≥4 kg |
|
Meropenem |
5 |
1 retrospective popPK study (n = 9) [95] 1 case report (n = 1) [96] 1 prospective popPK study with dosing simulations (n = 23) [97] 1 retrospective popPK study with dosing simulations (n = 26) [98] 1 prospective study in children with sepsis (n = 15) [99] |
0.03–15.6 y 2.7–59 kg |
CL is slightly lower [99], within [97], or higher [95][96][98] than the CL range observed in non-critically ill children, depending on the study population Increased dosing and extended infusion needed [95][96][97][98][99] |
|
Imipenem |
1 |
1 prospective study (n = 19) [100] |
9 d–12 y Body weight not reported |
Vd and CL comparable to non-critically ill children At least 100 mg/kg/day to avoid subtherapeutic concentrations |
|
Aztreonam |
1 |
1 case report (n = 1) [101] |
16 y |
CL double than reported in the package insert 2g q6h over 4h infusion achieved 40% fT>MIC |
|
Linezolid |
1 |
1 prospective popPK study with dosing simulations (n = 63) [102] |
0.1–15.3 y 4.2–70 kg |
Recommended age-differentiated dosing regimens lead to adequate attainment of the target AUC/MIC (>80) for sensitive pathogens Dose increase needed if MIC >1 mg/L Dose reduction needed if liver impairment (aspartate aminotransferase) |
|
Ciprofloxacin |
1 |
1 prospective study (n = 20) [103] |
3 m–4.75 y 4.2–21.2 kg |
No difference in CL and Vd between children aged <1 y and older. 20 mg/kg/day sufficient to cover pathogens with an MIC up to 0.8 mg/L 30 mg/kg/day in 3 doses needed in patients with normal renal function infected by pathogens with an MIC > 0.8 mg/L |
|
Daptomycin |
3 |
1 popPK study (n = 4) [104] |
8–14 y 17–45 kg |
Higher Vd and CL in sepsis patients vs. the patient without sepsis [104] CL in sepsis patients is double the CL in non-critically ill children [104] Children with sepsis showed suboptimal AUC values, even with increased dosing. This was even more pronounced in the burn patient. Increased dosing and TDM is recommended [104]. |
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics10101182
References
- Versporten, A.; Sharland, M.; Bielicki, J.; Drapier, N.; Vankerckhoven, V.; Goossens, H.; Members, A.P.G. The antibiotic resistance and prescribing in European Children project: A neonatal and Pediatric antimicrobial web-based point prevalence survey in 73 hospitals worldwide. Pediatr. Infect. Dis. J. 2013, 32, e242–e253.
- Gerber, J.S.; Newland, J.G.; Coffin, S.E.; Hall, M.; Thurm, C.; Prasad, P.A.; Feudtner, C.; Zaoutis, T.E. Variability in antibiotic use at children’s hospitals. Pediatrics 2010, 126, 1067–1073.
- Poole, N.M.; Shapiro, D.J.; Fleming-Dutra, K.E.; Hicks, L.A.; Hersh, A.L.; Kronman, M.P. Antibiotic Prescribing for Children in United States Emergency Departments: 2009–2014. Pediatrics 2019, 143.
- Brogan, T.V.; Thurm, C.; Hersh, A.L.; Gerber, J.S.; Smith, M.J.; Shah, S.S.; Courter, J.D.; Patel, S.J.; Parker, S.K.; Kronman, M.P.; et al. Variability in Antibiotic Use Across PICUs. Pediatr. Crit. Care Med. 2018, 19, 519–527.
- Hsieh, E.M.; Hornik, C.P.; Clark, R.H.; Laughon, M.M.; Benjamin, D.K., Jr.; Smith, P.B. Medication use in the neonatal intensive care unit. Am. J. Perinatol. 2014, 31, 811–821.
- Asin-Prieto, E.; Rodriguez-Gascon, A.; Isla, A. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J. Infect. Chemother. 2015, 21, 319–329.
- Nielsen, E.I.; Friberg, L.E. Pharmacokinetic-pharmacodynamic modeling of antibacterial drugs. Pharm. Rev. 2013, 65, 1053–1090.
- Cella, M.; Knibbe, C.; Danhof, M.; Della Pasqua, O. What is the right dose for children? Br. J. Clin. Pharm. 2010, 70, 597–603.
- Anderson, B.J.; Holford, N.H. Tips and traps analyzing Pediatric PK data. Paediatr. Anaesth. 2011, 21, 222–237.
- Le, J.; Bradley, J.S. Optimizing Antibiotic Drug Therapy in Pediatrics: Current State and Future Needs. J. Clin. Pharm. 2018, 58 (Suppl. 10), S108–S122.
- Downes, K.J.; Hahn, A.; Wiles, J.; Courter, J.D.; Vinks, A.A. Dose optimisation of antibiotics in children: Application of pharmacokinetics/pharmacodynamics in paediatrics. Int. J. Antimicrob. Agents 2014, 43, 223–230.
- Van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J. Clin. Pharm. 2018, 58 (Suppl. 10), S10–S25.
- Wilbaux, M.; Fuchs, A.; Samardzic, J.; Rodieux, F.; Csajka, C.; Allegaert, K.; van den Anker, J.N.; Pfister, M. Pharmacometric Approaches to Personalize Use of Primarily Renally Eliminated Antibiotics in Preterm and Term Neonates. J. Clin. Pharm. 2016, 56, 909–935.
- Allegaert, K.; Simons, S.H.P.; Tibboel, D.; Krekels, E.H.; Knibbe, C.A.; van den Anker, J.N. Non-maturational covariates for dynamic systems pharmacology models in neonates, infants, and children: Filling the gaps beyond developmental pharmacology. Eur. J. Pharm. Sci. 2017, 109, S27–S31.
- Thaden, J.T.; Chiswell, K.; Jaffe, I.; Bergin, S.P.; Yang, W.E.; Romaine, A.; Roberts, J.; Nambiar, S.; Farley, J.; Benjamin, D.K., Jr.; et al. Pediatric Antibacterial and Antifungal Trials From 2007 to 2017. Pediatrics 2018, 142.
- Schrier, L.; Hadjipanayis, A.; Stiris, T.; Ross-Russell, R.I.; Valiulis, A.; Turner, M.A.; Zhao, W.; De Cock, P.; de Wildt, S.N.; Allegaert, K.; et al. Off-label use of medicines in neonates, infants, children, and adolescents: A joint policy statement by the European Academy of Paediatrics and the European society for Developmental Perinatal and Pediatric Pharmacology. Eur. J. Pediatr. 2020, 179, 839–847.
- Van der Zanden, T.M.; Mooij, M.G.; Vet, N.J.; Neubert, A.; Rascher, W.; Lagler, F.B.; Male, C.; Grytli, H.; Halvorsen, T.; de Hoog, M.; et al. Benefit-Risk Assessment of Off-Label Drug Use in Children: The Bravo Framework. Clin. Pharm. 2021, 110, 952–956.
- Abdulla, A.; Edwina, A.E.; Flint, R.B.; Allegaert, K.; Wildschut, E.D.; Koch, B.C.P.; de Hoog, M. Model-Informed Precision Dosing of Antibiotics in Pediatric Patients: A Narrative Review. Front. Pediatr. 2021, 9, 624639.
- Smits, A.; Annaert, P.; Cavallaro, G.; De Cock, P.; de Wildt, S.N.; Kindblom, J.M.; Lagler, F.B.; Moreno, C.; Pokorna, P.; Schreuder, M.F.; et al. Current knowledge, challenges and innovations in developmental pharmacology: A combined conect4children Expert Group and European Society for Developmental, Perinatal and Paediatric Pharmacology White Paper. Br. J. Clin. Pharm. 2021, 1–20.
- Manolis, E.; Pons, G. Proposals for model-based paediatric medicinal development within the current European Union regulatory framework. Br. J. Clin. Pharm. 2009, 68, 493–501.
- Colin, P.J.; Allegaert, K.; Thomson, A.H.; Touw, D.J.; Dolton, M.; de Hoog, M.; Roberts, J.A.; Adane, E.D.; Yamamoto, M.; Santos-Buelga, D.; et al. Vancomycin Pharmacokinetics Throughout Life: Results from a Pooled Population Analysis and Evaluation of Current Dosing Recommendations. Clin. Pharmacokinet. 2019, 58, 767–780.
- Vancomycin FDA Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209481s000lbl.pdf (accessed on 26 August 2021).
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.G.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M.; et al. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur. J. Pharm. Sci. 2021, 162, 105812.
- Keij, F.M.; Kornelisse, R.F.; Hartwig, N.G.; Reiss, I.K.M.; Allegaert, K.; Tramper-Stranders, G.A. Oral antibiotics for neonatal infections: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2019, 74, 3150–3161.
- Friis-Hansen, B. Body water compartments in children: Changes during growth and related changes in body composition. Pediatrics 1961, 28, 169–181.
- Sethi, P.K.; White, C.A.; Cummings, B.S.; Hines, R.N.; Muralidhara, S.; Bruckner, J.V. Ontogeny of plasma proteins, albumin and binding of diazepam, cyclosporine, and deltamethrin. Pediatr. Res. 2016, 79, 409–415.
- Shaffer, S.G.; Bradt, S.K.; Hall, R.T. Postnatal changes in total body water and extracellular volume in the preterm infant with respiratory distress syndrome. J. Pediatr. 1986, 109, 509–514.
- Tréluyer, J.M.; Merlé, Y.; Tonnelier, S.; Rey, E.; Pons, G. Nonparametric population pharmacokinetic analysis of amikacin in neonates, infants, and children. Antimicrob. Agents Chemother. 2002, 46, 1381–1387.
- Lingvall, M.; Reith, D.; Broadbent, R. The effect of sepsis upon gentamicin pharmacokinetics in neonates. Br. J. Clin. Pharm. 2005, 59, 54–61.
- Smits, A.; Kulo, A.; Verbesselt, R.; Naulaers, G.; de Hoon, J.; Vermeersch, P.; Allegaert, K. Cefazolin plasma protein binding and its covariates in neonates. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3359–3365.
- Smits, A.; Pauwels, S.; Oyaert, M.; Peersman, N.; Spriet, I.; Saegeman, V.; Allegaert, K. Factors impacting unbound vancomycin concentrations in neonates and young infants. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1503–1510.
- Oyaert, M.; Spriet, I.; Allegaert, K.; Smits, A.; Vanstraelen, K.; Peersman, N.; Wauters, J.; Verhaegen, J.; Vermeersch, P.; Pauwels, S. Factors impacting unbound vancomycin concentrations in different patient populations. Antimicrob. Agents Chemother. 2015, 59, 7073–7079.
- Martin, E.; Fanconi, S.; Kälin, P.; Zwingelstein, C.; Crevoisier, C.; Ruch, W.; Brodersen, R. Ceftriaxone--bilirubin-albumin interactions in the neonate: An in vivo study. Eur. J. Pediatr. 1993, 152, 530–534.
- Rhodin, M.M.; Anderson, B.J.; Peters, A.M.; Coulthard, M.G.; Wilkins, B.; Cole, M.; Chatelut, E.; Grubb, A.; Veal, G.J.; Keir, M.J.; et al. Human renal function maturation: A quantitative description using weight and postmenstrual age. Pediatr. Nephrol. 2009, 24, 67–76.
- De Cock, R.F.; Allegaert, K.; Brussee, J.M.; Sherwin, C.M.; Mulla, H.; de Hoog, M.; van den Anker, J.N.; Danhof, M.; Knibbe, C.A. Simultaneous pharmacokinetic modeling of gentamicin, tobramycin and vancomycin clearance from neonates to adults: Towards a semi-physiological function for maturation in glomerular filtration. Pharm. Res. 2014, 31, 2643–2654.
- Cristea, S.; Krekels, E.H.J.; Allegaert, K.; Knibbe, C.A.J. The Predictive Value of Glomerular Filtration Rate-Based Scaling of Pediatric Clearance and Doses for Drugs Eliminated by Glomerular Filtration with Varying Protein-Binding Properties. Clin. Pharmacokinet. 2020, 59, 1291–1301.
- Cristea, S.; Krekels, E.H.J.; Rostami-Hodjegan, A.; Allegaert, K.; Knibbe, C.A.J. The Influence of Drug Properties and Ontogeny of Transporters on Pediatric Renal Clearance through Glomerular Filtration and Active Secretion: A Simulation-Based Study. AAPS J. 2020, 22, 87.
- Cristea, S.; Allegaert, K.; Falcao, A.C.; Falcao, F.; Silva, R.; Smits, A.; Knibbe, C.A.J.; Krekels, E.H.J. Larger Dose Reductions of Vancomycin Required in Neonates with Patent Ductus Arteriosus Receiving Indomethacin versus Ibuprofen. Antimicrob. Agents Chemother. 2019, 63, e00853-19.
- Allegaert, K.; Cossey, V.; Langhendries, J.P.; Naulaers, G.; Vanhole, C.; Devlieger, H.; Van Overmeire, B. Effects of co-administration of ibuprofen-lysine on the pharmacokinetics of amikacin in preterm infants during the first days of life. Biol Neonate. 2004, 86, 207–211.
- Marsot, A. Population pharmacokinetic models of first choice beta-lactam antibiotics for severe infections treatment: What antibiotic regimen to prescribe in children? J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. Soc. Can. Des. Sci. Pharm. 2020, 23, 470–485.
- Hartman, S.J.F.; Brüggemann, R.J.; Orriëns, L.; Dia, N.; Schreuder, M.F.; de Wildt, S.N. Pharmacokinetics and Target Attainment of Antibiotics in Critically Ill Children: A Systematic Review of Current Literature. Clin. Pharmacokinet. 2020, 59, 173–205.
- Hayton, W.L. Maturation and growth of renal function: Dosing renally cleared drugs in children. AAPS Pharmsci. 2000, 2, E3.
- Zembles, T.N.; Schortemeyer, R.; Kuhn, E.M.; Bushee, G.; Thompson, N.E.; Mitchell, M.L. Extended Infusion of Beta-Lactams Is Associated with Improved Outcomes in Pediatric Patients. J. Pediatr. Pharm. 2021, 26, 187–193.
- Chai, M.G.; Cotta, M.O.; Abdul-Aziz, M.H.; Roberts, J.A. What Are the Current Approaches to Optimising Antimicrobial Dosing in the Intensive Care Unit? Pharmaceutics 2020, 12, 638.
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153.
- Cies, J.J.; Moore, W.S., 2nd; Enache, A.; Chopra, A. β-lactam Therapeutic Drug Management in the PICU. Crit. Care Med. 2018, 46, 272–279.
- Balamuth, F.; Weiss, S.L.; Neuman, M.I.; Scott, H.; Brady, P.W.; Paul, R.; Farris, R.W.; McClead, R.; Hayes, K.; Gaieski, D.; et al. Pediatric severe sepsis in U.S. children’s hospitals. Pediatr. Crit. Care Med. 2014, 15, 798–805.
- Weiss, S.L.; Fitzgerald, J.C.; Pappachan, J.; Wheeler, D.; Jaramillo-Bustamante, J.C.; Salloo, A.; Singhi, S.C.; Erickson, S.; Roy, J.A.; Bush, J.L.; et al. Global epidemiology of Pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am. J. Respir. Crit. Care Med. 2015, 191, 1147–1157.
- Tamma, P.D.; Turnbull, A.E.; Milstone, A.M.; Hsu, A.J.; Carroll, K.C.; Cosgrove, S.E. Does the piperacillin minimum inhibitory concentration for Pseudomonas aeruginosa influence clinical outcomes of children with pseudomonal bacteremia? Clin. Infect. Dis. 2012, 55, 799–806.
- De Cock, P.A.J.G.; Desmet, S.; De Jaeger, A.; Biarent, D.; Dhont, E.; Herck, I.; Vens, D.; Colman, S.; Stove, V.; Commeyne, S.; et al. Impact of vancomycin protein binding on target attainment in critically ill children: Back to the drawing board? J. Antimicrob. Chemother. 2016, 72, 801–804.
- Seixas, G.T.F.; Araujo, O.R.; Silva, D.C.B.; Arduini, R.G.; Petrilli, A.S. Vancomycin Therapeutic Targets and Nephrotoxicity in Critically Ill Children with Cancer. J. Pediatr. Hematol. Oncol. 2016, 38, e56–e62.
- Giachetto, G.A.; Telechea, H.M.; Speranza, N.; Oyarzun, M.; Nanni, L.; Menchaca, A. Vancomycin pharmacokinetic-pharmacodynamic parameters to optimize dosage administration in critically ill children. Pediatr. Crit Care Med. 2011, 12, e250–e254.
- Gous, A.G.S.; Dance, M.D.; Lipman, J.; Luyt, D.K.; Mathivha, R.; Scribante, J. Changes in Vancomycin Pharmacokinetics in Critically Ill Infants. Anaesth. Intensive Care 1995, 23, 678–682.
- Bonazza, S.; Bresee, L.C.; Kraft, T.; Ross, B.C.; Dersch-Mills, D. Frequency of and Risk Factors for Acute Kidney Injury Associated with Vancomycin Use in the Pediatric Intensive Care Unit. J. Pediatr. Pharmacol. Ther. 2016, 21, 486–493.
- Cies, J.J.; Shankar, V. Nephrotoxicity in Patients with Vancomycin Trough Concentrations of 15–20 μg/ml in a Pediatric Intensive Care Unit. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 392–400.
- Silva, D.C.; Seixas, G.T.; Araujo, O.R.; Arduini, R.G.; Carlesse, F.A.; Petrilli, A.S. Vancomycin serum concentrations in Pediatric oncologic/hematologic intensive care patients. Braz. J. Infect. Dis 2012, 16, 361–365.
- Totapally, B.R.; Machado, J.; Lee, H.; Paredes, A.; Raszynski, A. Acute Kidney Injury During Vancomycin Therapy in Critically Ill Children. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 598–602.
- Goboova, M.; Kuzelova, M.; Kissova, V.; Bodakova, D.; Martisova, E. An adjustment of vancomycin dosing regimen for a young patient with augmented renal clearance: A case report/Úprava dávkového režimu vankomycínu pre mladého pacienta so zvýšeným renálnym klírensom: Kazuistika. Eur. Pharm. J. 2015, 62, 1–4.
- Thomas, C.A.; Picone, A.; Menon, S.; Willis, B.C. Empirical Vancomycin Dosing in Pediatric Patients with Congenital Heart Disease and the Impact of Cardiopulmonary Bypass on Trough Concentrations. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 1341–1346.
- Fitzgerald, J.C.; Zane, N.R.; Himebauch, A.S.; Reedy, M.D.; Downes, K.J.; Topjian, A.A.; Furth, S.L.; Thomas, N.J.; Scheetz, M.H.; Zuppa, A.F. Vancomycin Prescribing and Therapeutic Drug Monitoring in Children with and Without Acute Kidney Injury After Cardiac Arrest. Pediatr. Drugs 2019, 21, 107–112.
- Glover, M.L.; Cole, E.; Wolfsdorf, J. Vancomycin dosage requirements among Pediatric intensive care unit patients with normal renal function. J. Crit. Care 2000, 15, 1–4.
- Holsen, M.R.; Meaney, C.J.; Hassinger, A.B.; Fusco, N.M. Increased Risk of Acute Kidney Injury in Critically Ill Children Treated with Vancomycin and Piperacillin/Tazobactam. Pediatr. Crit. Care Med. 2017, 18, e585–e591.
- Maloni, T.M.; Belucci, T.R.; Malagutti, S.R.; Furtado, G.H.C. Describing vancomycin serum levels in Pediatric intensive care unit (ICU) patients: Are expected goals being met. BMC Pediatr. 2019, 19, 240.
- Avedissian, S.N.; Bradley, E.; Zhang, D.; Bradley, J.S.; Nazer, L.H.; Tran, T.M.; Nguyen, A.; Le, J. Augmented Renal Clearance Using Population-Based Pharmacokinetic Modeling in Critically Ill Pediatric Patients. Pediatr. Crit Care Med. 2017, 18, e388–e394.
- Zane, N.R.; Reedy, M.D.; Gastonguay, M.R.; Himebauch, A.S.; Ramsey, E.Z.; Topjian, A.A.; Zuppa, A.F. A Population Pharmacokinetic Analysis to Study the Effect of Therapeutic Hypothermia on Vancomycin Disposition in Children Resuscitated from Cardiac Arrest. Pediatr. Crit. Care Med. 2017, 18, e290–e297.
- Genuini, M.; Oualha, M.; Bouazza, N.; Moulin, F.; Treluyer, J.-M.; Lesage, F.; Renolleau, S.; Benaboud, S. Achievement of Therapeutic Vancomycin Exposure with Continuous Infusion in Critically Ill Children. Pediatr. Crit. Care Med. 2018, 19, e263–e269.
- Moffett, B.S.; Resendiz, K.; Morris, J.; Akcan-Arikan, A.; Checchia, P.A. Population Pharmacokinetics of Vancomycin in the Pediatric Cardiac Surgical Population. J. Pediatr. Pharmacol. Ther. 2019, 24, 107–116.
- Chen, Y.; Wu, D.; Dong, M.; Zhu, Y.; Lu, J.; Li, X.; Chen, C.; Li, Z. Population pharmacokinetics of vancomycin and AUC-guided dosing in Chinese neonates and young infants. Eur. J. Clin. Pharm. 2018, 74, 921–930.
- Sánchez, A.; López-Herce, J.; Cueto, E.; Carrillo, A.; Moral, R. Teicoplanin pharmacokinetics in critically ill paediatric patients. J. Antimicrob. Chemother. 1999, 44, 407–409.
- Reed, M.D.; Yamashita, T.S.; Myers, C.M.; Blumer, J.L. The pharmacokinetics of teicoplanin in infants and children. J. Antimicrob. Chemother. 1997, 39, 789–796.
- Lukas, J.C.; Karikas, G.; Gazouli, M.; Kalabalikis, P.; Hatzis, T.; Macheras, P. Pharmacokinetics of Teicoplanin in An ICU Population of Children and Infants. Pharm. Res. 2004, 21, 2064–2071.
- Aulin, L.B.S.; De Paepe, P.; Dhont, E.; de Jaeger, A.; Vande Walle, J.; Vandenberghe, W.; McWhinney, B.C.; Ungerer, J.P.J.; van Hasselt, J.G.C.; De Cock, P. Population Pharmacokinetics of Unbound and Total Teicoplanin in Critically Ill Pediatric Patients. Clin. Pharmacokinet. 2021, 60, 353–363.
- Zakova, M.; Pong, S.; Trope, A.; Atenafu, E.G.; Papaioannou, V.; Bitnun, S.A.; Richardson, S.; Schwartz, S.; Cox, P.; Parshuram, C.; et al. Dose Derivation of Once-Daily Dosing Guidelines for Gentamicin in Critically Ill Pediatric Patients. Ther. Drug Monit. 2014, 36, 288–294.
- Lopez, S.A.; Mulla, H.; Durward, A.; Tibby, S.M. Extended-interval gentamicin: Population pharmacokinetics in Pediatric critical illness. Pediatr. Crit. Care Med. 2010, 11, 267–274.
- Kraus, D.M.; Dusik, C.M.; Rodvold, K.A.; Campbell, M.M.; Kecskes, S.A. Bayesian forecasting of gentamicin pharmacokinetics in Pediatric intensive care unit patients. Pediatr. Infect. Dis. J. 1993, 12, 713–717.
- Sridharan, K.; Al Daylami, A. Clinical audit of gentamicin use by Bayesian pharmacokinetic approach in critically ill children. J. Infect. Chemother. 2020, 26, 540–548.
- Marik, P.E.; Havlik, I.; Monteagudo, F.S.E.; Lipman, J. The pharmacokinetics of amikacin in critically ill adult and paediatric patients: Comparison of once- versus twice-daily dosing regimens. J. Antimicrob. Chemother. 1991, 27, 81–89.
- Bressolle, F.; Gouby, A.; Martinez, J.M.; Joubert, P.; Saissi, G.; Guillaud, R.; Gomeni, R. Population pharmacokinetics of amikacin in critically ill patients. Antimicrob. Agents Chemother. 1996, 40, 1682–1689.
- Sherwin, C.M.T.; Wead, S.; Stockmann, C.; Healy, D.; Spigarelli, M.G.; Neely, A.; Kagan, R. Amikacin population pharmacokinetics among paediatric burn patients. Burns 2014, 40, 311–318.
- Wagner, B.P.; Pfenninger, J. Once daily dosing of netilmicin in neonatal and pediatric intensive care. Intensive Care Med. 1994, 20, 365–367.
- Nichols, K.; Chung, E.K.; Knoderer, C.A.; Buenger, L.E.; Healy, D.P.; Dees, J.; Crumby, A.S.; Kays, M.B. Population Pharmacokinetics and Pharmacodynamics of Extended-Infusion Piperacillin and Tazobactam in Critically Ill Children. Antimicrob. Agents Chemother. 2016, 60, 522–531.
- De Cock, P.; van Dijkman, S.C.; de Jaeger, A.; Willems, J.; Carlier, M.; Verstraete, A.G.; Delanghe, J.R.; Robays, H.; Vande Walle, J.; Della Pasqua, O.E.; et al. Dose optimization of piperacillin/tazobactam in critically ill children. J. Antimicrob. Chemother. 2017, 72, 2002–2011.
- Béranger, A.; Benaboud, S.; Urien, S.; Moulin, F.; Bille, E.; Lesage, F.; Zheng, Y.; Genuini, M.; Gana, I.; Renolleau, S.; et al. Piperacillin Population Pharmacokinetics and Dosing Regimen Optimization in Critically Ill Children with Normal and Augmented Renal Clearance. Clin. Pharmacokinet. 2019, 58, 223–233.
- Cies, J.J.; Shankar, V.; Schlichting, C.; Kuti, J.L. Population Pharmacokinetics of Piperacillin/Tazobactam in Critically Ill Young Children. Pediatr. Infect. Dis. J. 2014, 33, 168–173.
- Hartman, S.J.F.; Boeddha, N.P.; Ekinci, E.; Koch, B.C.P.; Donders, R.; Hazelzet, J.A.; Driessen, G.J.; de Wildt, S.N. Target attainment of cefotaxime in critically ill children with meningococcal septic shock as a model for cefotaxime dosing in severe pediatric sepsis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1255–1260.
- Von Hattingberg, H.M.; Marget, W.; Belohradsky, B.H.; Roos, R. Pharmacokinetics of cefotaxime in neonates and children: Clinical aspects. J. Antimicrob. Chemother. 1980, 6, 113–118.
- Beranger, A.; Oualha, M.; Urien, S.; Genuini, M.; Renolleau, S.; Aboura, R.; Hirt, D.; Heilbronner, C.; Toubiana, J.; Treluyer, J.M.; et al. Population Pharmacokinetic Model to Optimize Cefotaxime Dosing Regimen in Critically Ill Children. Clin. Pharmacokinet. 2018, 57, 867–875.
- Olguin, H.J.; Asseff, I.L.; Vieyra, A.C.; Pérez, A.G.; Saldaña, N.G.; Quesada, A.C.; Guillé, G.P. Effect of Severity Disease on the Pharmacokinetics of Cefuroxime in Children with Multiple Organ System Failure. Biol. Pharm. Bull. 2008, 31, 316–320.
- Hartman, S.J.F.; Upadhyay, P.J.; Hagedoorn, N.N.; Mathôt, R.A.A.; Moll, H.A.; van der Flier, M.; Schreuder, M.F.; Brüggemann, R.J.; Knibbe, C.A.; de Wildt, S.N. Current Ceftriaxone Dose Recommendations are Adequate for Most Critically Ill Children: Results of a Population Pharmacokinetic Modeling and Simulation Study. Clin. Pharmacokinet. 2021, 1–12.
- Butragueno-Laiseca, L.; Troconiz, I.F.; Grau, S.; Campillo, N.; Garcia, X.; Padilla, B.; Fernandez, S.N.; Santiago, M.J. Finding the Dose for Ceftolozane-Tazobactam in Critically Ill Children with and without Acute Kidney Injury. Antibiotics 2020, 9, 887.
- Cies, J.J.; Moore, W.S., 2nd; Enache, A.; Chopra, A. Ceftaroline for Suspected or Confirmed Invasive Methicillin-Resistant Staphylococcus aureus: A Pharmacokinetic Case Series. Pediatr. Crit. Care Med. 2018, 19, e292–e299.
- Jones, A.E.; Barnes, N.D.; Tasker, T.C.; Horton, R. Pharmacokinetics of intravenous amoxycillin and potassium clavulanate in seriously ill children. J. Antimicrob. Chemother. 1990, 25, 269–274.
- De Cock, P.A.; Standing, J.F.; Barker, C.I.; de Jaeger, A.; Dhont, E.; Carlier, M.; Verstraete, A.G.; Delanghe, J.R.; Robays, H.; De Paepe, P. Augmented renal clearance implies a need for increased amoxicillin-clavulanic acid dosing in critically ill children. Antimicrob. Agents Chemother. 2015, 59, 7027–7035.
- D’Agate, S.; Musuamba, F.T.; Della Pasqua, O. Dose Rationale for Amoxicillin in Neonatal Sepsis When Referral Is Not Possible. Front. Pharm. 2020, 11, 521933.
- Cies, J.J.; Moore, W.S., 2nd; Enache, A.; Chopra, A. Population Pharmacokinetics and Pharmacodynamic Target Attainment of Meropenem in Critically Ill Young Children. J. Pediatr. Pharm. 2017, 22, 276–285.
- Cies, J.J.; Moore II, W.S.; Calaman, S.; Brown, M.; Narayan, P.; Parker, J.; Chopra, A. Pharmacokinetics of Continuous-Infusion Meropenem for the Treatment of Serratia marcescens Ventriculitis in a Pediatric Patient. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, e32–e36.
- Rapp, M.; Urien, S.; Foissac, F.; Beranger, A.; Bouazza, N.; Benaboud, S.; Bille, E.; Zheng, Y.; Gana, I.; Moulin, F.; et al. Population pharmacokinetics of meropenem in critically ill children with different renal functions. Eur. J. Clin. Pharm. 2020, 76, 61–71.
- Saito, J.; Shoji, K.; Oho, Y.; Kato, H.; Matsumoto, S.; Aoki, S.; Nakamura, H.; Ogawa, T.; Hasegawa, M.; Yamatani, A.; et al. Population Pharmacokinetics and Pharmacodynamics of Meropenem in Critically Ill Pediatric Patients. Antimicrob. Agents Chemother. 2021, 65, e1909–e1920.
- Wang, Z.-M.; Chen, X.-Y.; Bi, J.; Wang, M.-Y.; Xu, B.-P.; Tang, B.-H.; Li, C.; Zhao, W.; Shen, A.-D. Reappraisal of the Optimal Dose of Meropenem in Critically Ill Infants and Children: A Developmental Pharmacokinetic-Pharmacodynamic Analysis. Antimicrob. Agents Chemother. 2020, 64, e00760-20.
- Giannoni, E.; Moreillon, P.; Cotting, J.; Moessinger, A.; Bille, J.; Decosterd, L.; Zanetti, G.; Majcherczyk, P.; Bugnon, D. Prospective determination of plasma imipenem concentrations in critically ill children. Antimicrob. Agents Chemother. 2006, 50, 2563–2568.
- Cies, J.J.; LaCoursiere, R.J.; Moore, W.S., II; Chopra, A. Therapeutic Drug Monitoring of Prolonged Infusion Aztreonam for Multi-Drug Resistant Pseudomonas aeruginosa: A Case Report. J. Pediatr. Pharmacol. Ther. 2017, 22, 467–470.
- Yang, M.; Zhao, L.; Wang, X.; Sun, C.; Gao, H.; Wang, X.; Qian, S. Population Pharmacokinetics and Dosage Optimization of Linezolid in Critically Ill Pediatric Patients. Antimicrob. Agents Chemother. 2021, 65, e02504-20.
- Lipman, J.; Gous, A.; Mathivha, L.; Tshukutsoane, S.; Scribante, J.; Hon, H.; Pinder, M.; Riera-Fanego, J.; Verhoef, L.; Stass, H. Ciprofloxacin pharmacokinetic profiles in paediatric sepsis: How much ciprofloxacin is enough? Intensive Care Med. 2002, 28, 493–500.
- Antachopoulos, C.; Ilia, S.; Kadiltzoglou, P.; Baira, E.; Dokoumetzidis, A.; Gikas, E.; Volakli, E.; Sdougka, M.; Briassoulis, G.; Roilides, E. Pharmacokinetics of Daptomycin in Critically Ill Pediatric Patients. Antimicrob. Agents Chemother. 2018, 62, e02462-17.
- Morris, S.; Gould, K.; Ferguson, L.P. The Use of Daptomycin to Treat Methicillin-Resistant Staphylococcus Epidermidis Bacteremia in a Critically Ill Child with Renal Failure. J. Pediatr. Pharmacol. Ther. 2017, 22, 300–303.
- Akins, R.L.; Haase, M.R.; Levy, E.N. Pharmacokinetics of Daptomycin in a Critically Ill Adolescent with Vancomycin-Resistant Enterococcal Endocarditis. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2006, 26, 694–698.
