Probiotics are beneficial active microorganisms that colonize the human intestines and change the composition of the flora in particular parts of the host. Recent evidence has shown that probiotics play significant roles in gut microbiota composition, which can inhibit the colonization of pathogenic bacteria in the intestine, help the host build a healthy intestinal mucosa protective layer, and enhance the host immune system. Based on the close relationship between the gut microbiota and human immunity, it has become an extremely effective way to improve human immunity by regulating the gut microbiome with probiotics.
1. Introduction
At the beginning of the 20th century, Nobel Prize winner Elie Metchnikoff proposed the concept of probiotics (meaning “for life”) [
1] and the modern definition of probiotics as “live microorganisms, when administered enough confers the health of the host”. Probiotics mainly exist in the human intestines and can play a beneficial role in the host by maintaining the balance of intestinal microbes [
2]. In daily life, common probiotics, such as
Lactobacillus or
Bifidobacterium, are usually consumed as active bacteria preparations [
3]. In the past few years, research on probiotics has made significant progress, and a large number of studies have proven that probiotics play an essential role in maintaining human health. For example, probiotics can play a role in the treatment of chronic inflammatory diseases, for example, Crohn’s disease. In addition, probiotics can also have an anti-cancer, anti-obesity, and anti-diabetes effect [
3]. There is growing evidence that probiotics can boost immunity and maintain health.
In general, probiotics are considered to be dietary factors that can influence the human gut microbiota and also have a regulatory effect on the composition and structure of the intestinal flora. It should be emphasized that the influence of intestinal flora on the immune system is vast. In the human body, intestinal flora can maintain the integrity of the mucosal barrier, provide nutrients, and resist pathogens [
11]. Interestingly, recent research has found that the immune system plays a role in the relationship between sleep and skin character [
12]. In addition, the central nervous system (CNS) is also affected by the immune system [
13]. Accordingly, immunity directly affects people’s lives, and using probiotics to regulate intestinal bacteria has become an effective way to improve immunity.
2. The Mechanisms of Probiotics to Exert Their Beneficial Effects
2.1. Regulation of Intestinal Flora by Probiotics
As research progresses, the regulation of intestinal flora is effective in boosting immunity, treating metabolic disorders, and even treating mental illnesses [
15]. As a class of beneficial microorganisms, probiotics also play a massive role in regulating intestinal flora [
2]. According to Bagarolli et al. [
16], mice fed a high-fat diet showed significant alterations in the intestinal flora associated with a range of diseases. Injecting probiotics into obese animals revealed that the Firmicutes of the intestinal flora decreased and the Actinobacteria increased. Treatment with probiotics can reverse intestinal flora dysbiosis and treat inflammatory responses in mice. Intestinal flora is a critical point in treating inflammatory diseases. We can infer that the regulation of intestinal flora by probiotics can effectively treat related inflammatory disorders. Researchers found that compared to the control group, the beneficial bacteria (such as Oscillibacter and Prevotella) and the concentrations of SCFAs in the intestinal flora of the probiotic-treated mice increased significantly [
17]. SCFAs are of great research value as important metabolites of intestinal microorganisms. Today, SCFAs can enhance immunity [
18] and act as a chemical signal for brain–gut communication [
19]. By using probiotics, the number of SCFAs producing microorganisms in the gut can be increased. By doing so, this can have a beneficial effect on our immune system and maintain a healthy lifestyle.
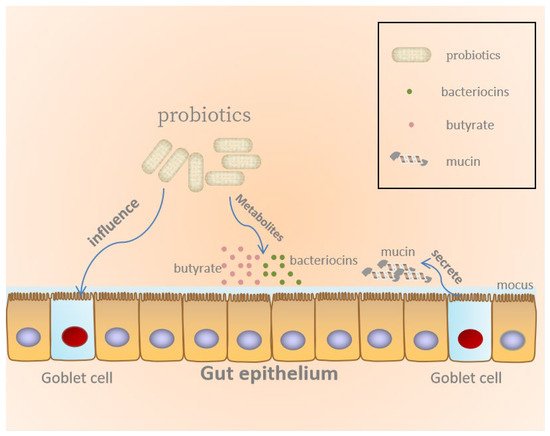
2.2. Maintenance of the Epithelial Barrier
There is substantial evidence that probiotics can strengthen the intestinal barrier, regulate mucosal immune function, and produce metabolites beneficial to the host to maintain health, as illustrated in
Figure 1 [
22,
23].
Figure 1. Probiotics maintain gut epithelial barrier.
2.3. Inhibition of Pathogen by Probiotics
There are a large number of microorganisms in the human intestine, including pathogenic microorganisms and probiotics. The homeostasis of the gut microbiota may be altered by pathogenic microorganisms, which can elevate the risk of developing related diseases [31]. In past studies, it has been well documented that probiotics can protect the intestinal tract by inhibiting pathogenic bacteria. Therefore, the effects of probiotics on inhibiting pathogens in the gut and their underlying mechanisms have received significant attention from the research community. There are many mechanisms for probiotics to inhibit pathogenic microorganisms, such as stimulation of epithelial barrier function, producing antimicrobial substances, limiting access of pathogenic microorganisms to nutrient resources, and competitive exclusion by competition for binding sites [32,33]. Conversely, a crucial beneficial mechanism of probiotics is the competitive exclusion of pathogens [30]. For example, the experiment by Fang et al. [34] showed that probiotic Escherichia coli Nissle 1917 (EcN) can secrete DegP (a bifunctional periplasmic protein) to inhibit enterohemorrhagic E. coli (EHEC). The probiotic Escherichia coli outcompetes pathogenic biofilms via extracellular DegP activity during dual-species biofilm formation. In another experiment, probiotics can secrete antibacterial substances, causing steric hindrance and competitive adhesion sites and nutrients to prevent Helicobacter pylori from binding to epithelial cells [35]. On the other hand, secreting antibacterial compounds is another vital function of probiotics. A typical example is that probiotics can secrete organic acids during carbohydrate fermentation, such as butyric acid, acetic acid, and propionic acid. Organic acids have been considered to be the main antimicrobial compounds responsible for their inhibitory activity against pathogens. The decrease in pH and the presence of undissociated acid make organic acids have a certain antibacterial activity [30]. Interestingly, some experiments also found that through inhibition of the pathogen signal system, intestinal pathogens can be eliminated.
2.4. The Modulation and Proper Maturation of the Immune System
Probiotics have a certain influence on IECs. Bacterial fragments of probiotics can be internalized into IECs, activate related immune cells, and promote IECs to start a complex signal network. Its main function is to activate the innate response and the cytokines released by T cells and stimulate immune cells related to lamina propria. The results showed that probiotics could activate the immune system [
23,
37].
Probiotics promote the maturation of humoral immune mechanism. Probiotics entering the intestine can stimulate the production of the IgA antibody [
39]. Studies have found that oral probiotics can effectively increase IgA
+ cells in intestinal lamina propria. Many studies have shown that probiotics can induce the IgA cycle, strengthen and maintain the immune surveillance of the mucosa away from the intestine, and promote the maturation of the humoral immune mechanism [
23,
28,
40].
Probiotics can increase the number of macrophages and dendritic cells (DCs) in lamina propria and enhance their function in the intestine for a period of time. DCs are mainly responsible for recognizing and eliminating exogenous pathogens in the immune system, and are the primary cell type involved as “sensors” of microbial ligands through activation of innate immune receptors. DCs can enter the mucosa-associated lymphoid tissue or discharge lymph nodes continuously through the antigen barrier [
23,
40,
41,
42]. Macrophages are also important immune cells. Macrophages are mainly responsible for phagocytosis of cell debris and pathogens, the activation of lymphocytes and other immune cells against pathogens, and the fixation of free cells. Recently, there are many studies on the interaction between probiotics and macrophages. Among them, one study showed that
Lactobacillus probiotic strains activated the in vitro inflammatory response of macrophages via the synthesis of proinflammatory mediators, including cytokines, reactive oxygen species (ROS), and participation in the signaling cascades, such as the nuclear factor kB (NF-kB) and Toll-like receptor 2 (TLR2) pathways [
39].
3. Effect of Gut Microbiome on Immunity
3.1. The Role of Gut Microbiome in the Immune System
As is known, the gut has an immune system that can prevent the invasion of pathogenic microorganisms and create a suitable environment for beneficial bacteria, which plays a crucial role in maintaining host health. Many studies have proven that the intestinal microbiome can strengthen the intestinal immune system [25,42], indicating intestinal flora plays an important role in the immune system.
3.2. Gut Microbiome, Food Allergy, Cancer and Depression
The study confirmed that in multiple interconnected networks of host immunity and homeostasis, the gut microbiota plays an important role in maintaining homeostasis [56]. The gut microbiota is involved in structural modification of the host intestinal mucosa, neurotransmission, vitamin K production, as well as the development of immune responses [57]. Currently, many experiments have suggested a role for the gut microbiota in the pathogenesis and progression of food allergy [53], and gut microbes can function to maintain the immune system’s efficiency [58].
The origin of cancer is a malignant tumor of epithelial tissue; the generation of cancer is generally thought to be secondary to a state of local chronic inflammation [62]. The occurrence of cancer is directly related to immune deficiency. With the discovery of the beneficial effect of intestinal flora on immunity, the relationship between the intestinal microbiome and cancer has been paid more and more attention [63]. The most typical example is the relationship between intestinal flora and colorectal cancer. Colorectal cancer has a high incidence and mortality [64,65]. Through research in recent years, it has been proven that there is a close relationship between intestinal microorganisms and colorectal cancer. The study found that specific species of bacteria may affect both the risk of colorectal cancer and the growth of existing tumors [66]. For instance, the experiment by Ahn et al. [67] showed that microbial diversity was significantly lower in the gut of patients with colorectal cancer. More specifically, colorectal cancer patients had higher numbers of Fusobacterium and Porphyromonas and a lower relative abundance of clostridia. This result has also been confirmed in other experiments; other studies have found that survival of patients with colorectal cancer is associated with the concentration of Fusobacterium nucleatum [64].
The common beneficial microorganisms in the gut include Bifidobacterium sp., Streptococcus thermophilus, Lactobacillus sp., and Saccharomyces boulardii [69]. They have become a regular part of our dietary habits [70]. The gut microbiome may influence normal brain development, mood, and pain sensitivity. Moreover, several research findings have shown that probiotics can affect the CNS by regulating the intestinal flora and preventing mental diseases through the gut–brain axis [71,72,73]. Recently, it has been proven that these beneficial microorganisms in the intestine can enhance immunity, and the improvement of immunity helps prevent the occurrence of depression. Depression is the most common disorder. The main clinical manifestations are depression, slow thinking, and impaired cognitive function. With the continuous development of science and technology, metagenomics and molecular tools have been further improved, contributing to the continued promotion of research [74]. In studies of depressed patients, it was found that cell-mediated activation of adaptive immunity appears to be dramatically different in depressed patients compared to the general population [75]. Moreover, in past studies, there have been a large number of experimental results that have proven that the activation of innate immune mechanisms, such as the activation of the proinflammatory cytokines interleukin-6 and interleukin-1, has a certain association with the development of depression [76]. The link between the occurrence of depression and the immune system is obvious. The intestinal flora has the function of enhancing immunity and refining the immune system, which has a certain theoretical basis for advocating that intestinal flora can improve immunity to treat depression.
4. Probiotics, Gut Microbiome, Immunity and Life
4.1. The Role of Gut Microbiome in Obesity and Local Inflammation of Adipose Tissue
Obesity is a common metabolic disease. There are many factors that cause obesity. In addition to lifestyle, diet, and genetic factors, some studies have suggested that a cause of obesity could also be disorders of the intestinal flora [
77,
78,
79]. Moreover, patients with obesity are more likely to have local inflammation in adipose tissue than healthy lean individuals, and then, the local inflammation can turn into systemic inflammation [
80]. Intestinal flora can prevent local inflammation of adipose tissue by preventing obesity and enhancing immunity. It can be seen from the above that there is an interactive relationship between intestinal flora and the immune system, and the intestinal flora plays an important role in the immune system. In addition, it is well established that the gut microbiota can influence the development of obesity. Using genetic sequencing of fecal samples from multiple obese patients, researchers identified different strains among them and compared them with lean volunteers. They found that obese individuals had significantly fewer Bacteroidetes and more Firmicutes [
81], which means the incidence of obesity may be related to the proportion of Firmicutes and Bacteroidetes [
82].
4.2. The Relationship between Immunity and Sleep and the Effect of Gut Microbiome on Sleep
In recent years, studies have shown that sleep is closely related to the diversity of the intestinal flora. For example, Smith et al. [
90] demonstrated that the diversity of the gut microbiota would decrease because of sleep fragmentation, but increase due to good sleep quality and enough sleep time. They also found that short-term sleep control has little effect on intestinal microbial diversity, but intestinal microbial diversity can have a long-term impact on sleep quality. In summary, the experiment concluded that the diversity of the gut microbiome promotes healthier sleep. Moreover, another study found that transplanting fecal microbes to regulate gut microbes can improve sleep efficiency. This experiment has shown that the regulation of intestinal flora may lead to novel sleep intervention strategies [
90]. Conversely, sleep can also affect intestinal flora. A study in mice found shifts in the microbiome after longer-term sleep fragmentation [
91]. More specifically, the treatment with
Lactobacillus casei strain Shirota (LcS) causes a positive impact on patients’ sleep duration and quality of sleep [
92]. These results all proved that there is a close relationship between sleep and intestinal flora.
4.3. The Relationship between Intestinal Flora and Skin and the Use of Probiotics to Improve Skin Quality
Up to now, there have been many experiments demonstrating a bidirectional link between the skin and the gut, and the character of some gastrointestinal disorders can be manifested through the skin. With the continuous research on the relationship between intestinal microorganisms and host health, scientists are now investigating how local microbes influence the immune competence of distant organs. Among them, the most focus has been on how gut microbes affect lung, heart, skin and other organs [
93]. Therefore, researchers found that the intestinal microbiome is closely related to common skin diseases, such as acne, psoriasis, and atopic dermatitis (AD) [
94]. Moreover, in addition to some traditional methods, the use of probiotics in treating these diseases has been paid more and more attention. Take the relationship between intestinal microbiome and AD as an example. AD is a common chronic inflammatory skin disease in the world [
95]. Nowadays, the mainstay of AD treatment is to use anti-inflammatory and emollients, by which the disadvantages of poor immune tolerance and barrier dysfunction are compensated. In recent years, studies have found that probiotics can play a role in preventing and treating AD by enhancing immunity [
94]. For example, one study in Norway found that the incidence rate of AD can be effectively reduced by supplying probiotic milk to women and infants before and after delivery [
94]. In another study, it was found that compared with the healthy control group, the level of
Bifidobacterium in the intestinal tract of AD patients was lower, and the level of
Bifidobacterium in the intestinal tract was negatively correlated with the severity of disease in AD patients. The study also showed that the changing of intestinal flora may be earlier than the development of AD. Accordingly, we can infer that intestinal flora disorders may be one of the reasons for the occurrence of AD [
95].
5. Conclusions
As a kind of beneficial microorganism, probiotics can regulate the composition of intestinal flora and enhance immunity. Probiotics can improve host immunity by maintaining the epithelial barrier, inhibiting pathogens from adhering to the intestinal surface, and modulating and properly maturing the immune system. Moreover, probiotics can also improve host immunity by affecting intestinal flora to treat certain diseases. Nowadays, it has been proven that there is a close relationship between probiotics, intestinal flora and immunity. In the future, the mechanism by which probiotics regulate the structure of intestinal flora and improve immunity will be further elucidated, and will also be an effective way to improve people’s quality of life.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26196076