1. Introduction
Escherichia coli (
E. coli) is a gram-negative bacteria and causative agent of many infectious diseases in humans. Many bacterial infections such as urinary tract infections, bloodstream infections, pneumonia, surgical site infections [
1,
2,
3], bacterial sepsis [
4,
5], and neonatal bacterial meningitis are mainly produced by
E. coli [
6].
The Gram-negative bacteria are characterized by their cell envelopes, which are composed of a thin peptidoglycan cell wall sandwiched between an inner cytoplasmic cell membrane and a bacterial outer membrane (OM) [
7,
8]. The OM is an additional protection layer that prevents several substances from entering the bacterium. Nevertheless, OM comprises channels named porins, which allow access to numerous molecules such as drugs [
9]. The OM of Gram-negative bacteria is the leading cause of resistance for a wide range of antibiotics such as β-lactams, quinolones, and other antibiotics [
10]. Most antibiotics must pass through OM for effective targeting [
11]. Hydrophobic molecules can penetrate through the diffusion pathway; in contrast, hydrophilic antibiotics, including β-lactams can pass via porins. Any variation in the OM by Gram-negative bacteria, including mutations in porins, can cause resistance [
12].
The use of antibiotics is an efficient, prevailing, and the utmost method for treating
E. coli infections. However, huge numbers of drug-resistance strains have appeared due to antibiotics misuse in the last 50 years [
13,
14]. Furthermore, the inappropriate and overuse of antimicrobial agents has increased pathogens and humans’ resistance [
15]. Numerous antibacterial agents such as ampicillin, cotrimoxazole, azithromycin, and gentamicin for
E. coli therapy have been revealed to stimulate the Shiga toxin release from
E. coli [
16]. In addition, the antibodies treatment is an effective method for deactivating the virulence factors and toxins from
E. coli [
17]. Still, the specificity of antibodies is a major challenge for treating
E. coli infections using antibodies [
18]. Vaccine therapy using inactivated
E. coli has been used to robust the immune responses in humans. However, the short duration of the vaccine producing immunity against bacterial infections is a major drawback for treating
E. coli [
19]. Despite this, antibiotics-based therapy is still the main strategy against bacterial infections. There is a need to discover new antibacterial agents with new mechanisms to combat resistant bacterial strains [
20].
Conventional methods have been used for the diagnosis of
E. coli infections for several years, including enzyme-linked immune sorbent assay (ELISA) and polymerase chain reaction (PCR) [
21]. The non-culturing approaches are conducted by staining the urine sample for the detection of bacterial infections, but these approaches are time-consuming with less precision value [
22]. Meanwhile, the culturing method is one of the oldest techniques for detecting infectious bacteria. Few drawbacks accompany this method, e.g., preparation of individual culture medium to detect each microorganism in the sample for optimal growth [
23]. PCR-based methods have been utilized for the identification and diagnosis of bacterial infections [
24]. A multiplex PCR test has been established to recognize
E. coli producing bacterial infections [
25]. ELISA is also one of the molecular techniques widely used to detect bacterial components in the sample [
26]. Nevertheless, the prolonged incubation period, extensive sample cleaning, and purification of biomolecules are major disadvantages of these methods [
27,
28]. To tackle limitations related to the approaches mentioned above, nanotechnology is a quick, efficient and versatile solution for treating and detecting bacterial infections [
29]. Recently, numerous NPs, such as silver NPs, zinc oxide NPs, and cationic surfactant NPs, have been used for bacterial infection treatment [
30,
31,
32]. The antibacterial potential of silver NPs generally depends on the particle size, shape and surface modification [
33,
34,
35,
36]. The loading of antibacterial moiety into the silver NPs also enhances its antimicrobial activity [
37,
38]. Zinc oxide is a multifunctional inorganic material that has been used widely in optoelectronic devices, textiles, cosmeceuticals, and most importantly, as an antibacterial agent [
39]. The cationic surface NPs are positively charged and can kill bacteria by disrupting bacterial cell wall/membrane, generating free radicals [
40,
41].
Nanotechnology-based approaches such as gold NPs, silver NPs, magnetic NPs, and quantum dots (QDs) reveal selective target-binding characteristics [
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57]. These characteristics make them ideal candidates for the diagnosis and biosensing of
E. coli infections [
58,
59,
60,
61,
62,
63]. The binding to specific ligands such as antibodies and enzymes for detecting bacterial infections is due to the surface properties of NPs. This boosts the specificity of the nanosensor being developed [
64]. The entrapment of NPs into nanosensors also enhances the rapid detecting ability of the portable device. NPs, portable devices, nanotubes, nanowires, and nanomechanical devices are typical examples of functional probes for the detection and disinfection of pathogens and other contaminants in different mediums [
65,
66,
67].
2. Point of Care (POC) Devices for Clinical Applications
Pathogens, and all diseases associated with them, are a significant concern worldwide [
129]. Diagnostic tests have been suggested to prolong the effectiveness of current antimicrobials; culture and other conventional diagnostics are hindered in their practicality as they are time- and labour-intensive to perform. POC testing is performed near where the patient is being treated and can provide timely results that allow evidence-based clinical interventions to be made (
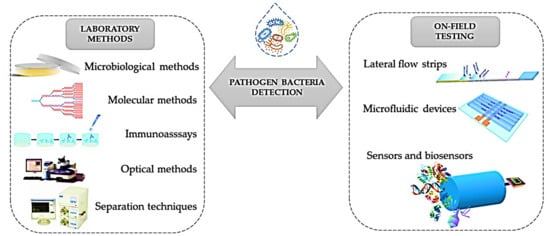
Figure 11) [
130]. For example, a portable multiplexed bar-chart SpinChip (MB-SpinChip) integrated with NP-mediated magnetic aptasensors was developed for visual, quantitative instrument-free detection of multiple pathogens. This versatile multiplexed SpinChip combines aptamer-specific recognition and NP-catalysed pressure amplification to achieve a sample-to-answer output for sensitive point-of-care testing (POCT). This user-friendly MB-SpinChip allows visual, quantitative detection of multiple pathogens simultaneously with high sensitivity, but without utilizing any specialized instruments. Using this MB-SpinChip, three major foodborne pathogens, including
Salmonella enterica, Escherichia coli, and
Listeria monocytogenes, were specifically quantified in apple juice with limits of detection of about 10 CFU/mL [
131]. In another study, a smartphone-based nanosensor was developed to detect zika virus (ZIKV) infection. In this light, a nanomotor-based bead-motion cell phone (NBC) system was developed for the immunological detection of ZIKV. The presence of a virus in a testing sample results in the accumulation of platinum (Pt)-nanomotors on the surface of beads, causing their motion in H
2O
2 solution. Then, the virus concentration is detected in correlation with the change in beads motion. The developed NBC system could detect ZIKV in samples with virus concentrations as low as 1 particle/μL. The NBC platform technology has the potential to be used in the development of point-of-care diagnostics for pathogen detection and disease management in developed and developing countries [
132]. Of course, new simulation and machine learning approaches can help better optimize these devices [
133,
134,
135]. The schematic representation of the current analytical methods and POC devices applied for the detection of E. coli are shown in
Figure 1.
Figure 1. Schematic representation of the current analytical methods and POC devices applied to detect
E. coli Reprinted from ref. [
129].
3. Regulatory Landscape of Nanotechnology in Biomedical Applications
The safety assessment of medical devices containing or deriving from nanotechnology is carried out by the US-FDA’s Centre for Devices and Radiological Health (CDRH), housing a Nanotechnology Regulatory Science Research Programme that is based on three pillars: physicochemical characterization methods, in vitro and in vivo models, and (toxicological) risk assessment [
136,
137]. The types of devices that incorporate nanotechnology include antimicrobial, dental, orthopaedic, neurological, and combination devices and in vitro diagnostic tools. They use various nanomaterials, including silver, zirconia, titanium and titanium dioxide, iron oxides, polymers, gold, graphene etc. Safety assessment of such medical devices should encompass the determination of the rate and magnitude of the nanomaterials into the body for which fit-for-the-purpose in vitro tests would be desirable [
138]. Moreover, advanced toxicological risk assessment approaches should support the understanding that the release and patient exposure results in adverse health impacts. It is important to know whether NPs affect the accuracy and/or reliability of standard biocompatibility or toxicity test assays, such as cytotoxicity and genotoxicity. Because of the vast number of sizes, shapes, and chemistry of nanomaterials, there is the need for the development of in vitro models (2D, 3D, organ on a chip, organoids) and in silico models in order to predict human responses and improve in vitro to in vivo extrapolations [
137].