Adenoviruses represent exceptional candidates for wide-ranging therapeutic applications, from vectors for gene therapy to oncolytics for cancer treatments. The first ever commercial gene therapy medicine was based on a recombinant adenovirus vector, while most recently, adenoviral vectors have proven critical as vaccine platforms in effectively controlling the global coronavirus pandemic.
1. Introduction
Adenoviruses (AdVs) can cause mild health problems, like acute respiratory, gastrointestinal, and ocular infections, but have no oncogenic potential in humans [
1]. To ensure viral progeny, AdVs need to efficiently infect permissive cells that will enable both adenoviral entry and replication of DNA. The life cycle of AdVs can be simply divided into several stages: binding to a receptor expressed on a cell surface, entry into the cell by endocytosis, endosomal escape, trafficking through the cytoplasm, and docking at the nuclear pore complex (NPC), followed by nuclear entry of the adenoviral genome. Once adenoviral DNA enters the nucleus, expression of early and late genes allows assembly of new viral particles and their release from the host cell is enabled.
Thanks to their features, like the relative ease with which their genome can be modified as well as relative tolerance towards genetic manipulation, AdVs have been recognized as promising vectors for gene transfer. Today, according to the Wiley database on Gene Therapy Trials Worldwide, AdVs present 17.5% of vectors used in gene therapy clinical trials (
https://a873679.fmphost.com/fmi/webd/GTCT; accessed on 10 August 2021). The first ever commercial gene therapy medicine was recombinant adenovirus encoding human p53 tumor suppressor gene, Gendicine, registered for the treatment of head and neck cancers on the market in China [
2]. Besides being vectors for gene transfer, AdVs are widely used as vectors for vaccination. Numerous AdV vaccine candidates for treating infectious diseases and cancer are under investigation, with the most important milestone being achieved when European Medicines Agency recently approved three AdV-vector-based vaccines: one against Ebola (Zabdeno; Ad26.ZEBOV [
3]) and two against Covid-19 (ChAdOx1 nCoV-19 [
4] and Ad26.COV2.S [
5]), demonstrating the efficacy of AdV-based vaccines as tools for controlling pandemic outbreaks.
We distinguish the two types of commonly used adenoviral vectors: replication-defective and oncolytic adenoviruses. Due to molecular engineering, replication-defective AdVs cannot replicate in infected cells, however they provide high expression of transgenes with minimum expression of AdV proteins. Unlike replication-defective AdVs (also known as vectors), oncolytic AdVs selectively replicate in and lyse cancer cells, and are, therefore, commonly used vectors in clinical trials for cancer gene therapy [6]. The world first oncolytic virus product was also an adenovirus, Oncorine (H101), an oncolytic adenovirus intended to be used in combination with chemotherapy as a treatment for patients with late-stage refractory nasopharyngeal cancer [7]. Currently, a large number of AdV platforms are being developed for oncology applications, for example, Enadenotucirev, a clinical stage oncolytic AdV with broad potential to target solid tumor carcinomas [8]; DNX-2401; Delta-24-RGD oncolytic AdV, developed as immunotherapeutic treatment for recurrent malignant glioma [9]; and TILT-123, a human AdV5/AdV3 chimeric oncolytic adenovirus encoding for tumor necrosis factor alpha and human interleukin 2 aimed at generating an anti-cancer immune response [10].
To fulfil its role as a vector, an AdV needs to successfully deliver its DNA genome to the host nucleus, a process highly influenced by AdV intracellular translocation. In this review, we discuss which factors are involved in AdV endocytosis, focusing mainly on receptors and endocytic scaffold proteins, as well as the influence of a particular type of endocytosis on AdV intracellular trafficking.
Human adenoviruses (HAdVs) are non-enveloped double stranded DNA viruses with icosahedral capsids of approximately 90 nm in diameter and mass of ~150 megadaltons [
11]. HAdVs consist of 13 structural proteins that form the outer capsid of icosahedral geometry and the inner core, where double-stranded DNA is bound with the core proteins. Major building blocks of the HAdV capsid are hexon and penton. There are 240 copies of the hexon trimer, and 12 pentons comprising an extended fiber protein non-covalently attached to the penton base protein. The fiber protein is composed of tail, shaft, and knob domains, the latter containing several loops. In addition, capsids comprise minor proteins IIIa, VI, VIII, and IX [
12]. HAdVs are classified into seven subgroups, A–G [
13], according to hemagglutination and serum neutralization reactions or by genome sequencing and bioinformatics analysis (International Committee on Taxonomy of Viruses—ICTV). HAdV infection starts with binding to the primary receptor at the cell surface. Primary receptor usage differs between different HAdVs serotypes. After initial attaching to the primary receptor, HAdV binds αv integrins on the cell surface through an RGD peptide sequence present in the penton base of the virus [
14]. This interaction triggers internalization of HAdV, as well as a number of signaling events that lead to virus cell entry within the endosomal compartment [
15]. Partial disassembly of the capsid occurs prior to membrane penetration, however the escape of incoming adenoviruses from endosomes is dependent on the viral membrane lytic protein VI, whose lytic activity is pH-independent [
16,
17]. Once liberated from the endosome, the fibreless HAdV capsid is transported towards the nucleus along a network of microtubules [
18] and motor proteins, such as dynein (unidirectional movement) or kinesin (bidirectional) [
19,
20]. Final docking of the HAdV capsid occurs at the nuclear pore and the HAdV genome enters the nucleus [
21], where it remains episomal. A scheme of HAdV cell entry is presented on
Figure 1.
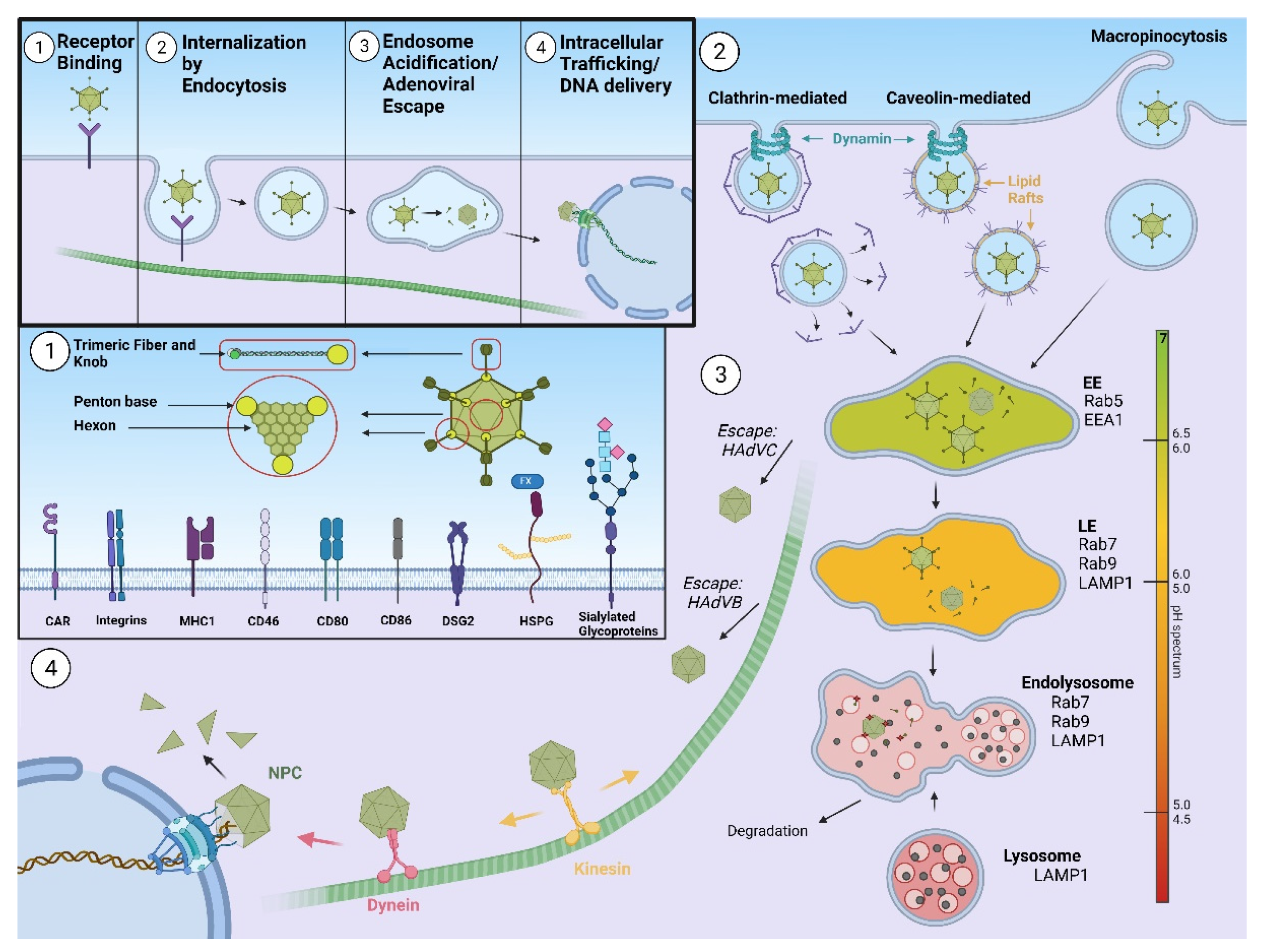
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13101585

