Biofilms comprising aggregates of microorganisms or multicellular communities have been a major issue as they cause resistance against antimicrobial agents and biofouling. To date, numerous biofilm-forming microorganisms have been identified, which have been shown to result in major effects including biofouling and biofilm-related infections. Quorum sensing (which describes the cell communication within biofilms) plays a vital role in the regulation of biofilm formation and its virulence. As such, elucidating the various mechanisms responsible for biofilm resistance (including quorum sensing) will assist in developing strategies to inhibit and control the formation of biofilms in nature. Employing biological control measures (such as the use of bioactive compounds) in targeting biofilms is of great interest since they naturally possess antimicrobial activity among other favorable attributes and can also possibly act as potent antibiofilm agents.
1. Introduction
Biofilms are defined as aggregates of microorganisms or multicellular communities that are embedded in extracellular matrix produced by the microorganisms themselves [
1,
2]. Some biofilms contain only a single species, while some others contain a multitude of species [
3]. The matrix in which microorganisms are encased is composed of extracellular polymeric substances (EPS) typically consisting of polysaccharides, proteins or peptides, lipids, as well as deoxyribonucleic acids (DNA). These biofilm components facilitate the coherence of cells and cell surface attachment [
4,
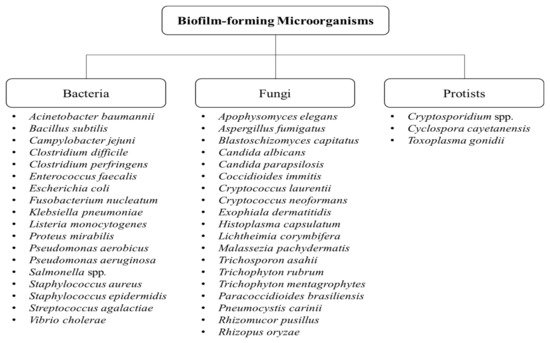
5]. Biofilm formation is not only attributed to bacteria, but also to fungi and protists [
3,
6]. Some of the examples of biofilm-forming bacteria, fungi, and protists are shown in
Figure 1.
Figure 1. Types of biofilm-forming microorganisms [
3,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17].
Biofilms can be formed on any surfaces, including both biotic and abiotic surfaces [
2]. Biotic surfaces such as the teeth of animals, a most common sites for bacteria to form biofilm. Biofilm formation on abiotic surfaces is prevalent in industrial and medical settings, whereby they are evidently found in industrial pipes and equipment for clinical use [
2]. Among all the microorganisms, bacterial biofilm is highly associated with nosocomial diseases due to the colonization of bacteria on the surface of medical equipment, most commonly seen in indwelling urinary catheters and implantable medical devices [
18,
19].
Biofilm formation is the protective mechanism of microorganisms that is involved in stress coping [
20]. Their survival can be prolonged in unfavourable conditions as they are able to withstand the stress when they are within biofilms. This does not happen to planktonic cells as they can easily die in such conditions [
20]. The presence of any stress can trigger the formation of biofilm and cause free-floating (planktonic) bacteria to switch to the biofilm mode of growth [
21]. Some common stressful conditions that are faced by the organisms include nutrient deprivation, changes in pH, and the presence of antibiotics in their surroundings, which are discussed later in this review [
20].
The formation of biofilms in the human body is associated with high morbidity and mortality rates [
18]. One of the reasons is because they are capable of resisting phagocytosis. Therefore, the clearing of biofilms away from the host is of great difficulty, allowing them to persist and cause chronic infections [
22]. A common example is the formation of dental plaque, a type of bacterial biofilm that forms on the teeth, responsible for tooth decay and gum-related diseases [
23]. Besides that, diseases caused by biofilm-forming organisms are very difficult to treat due to the organisms’ increased resistance against antibiotics. As such, it is a great challenge for researchers and physicians to search for alternative compounds or substances to target biofilms [
19]. In this review, we focused on the uses of various bioactive compounds in targeting biofilm, alongside with the factors triggering and contributing to biofilm formation and its impact.
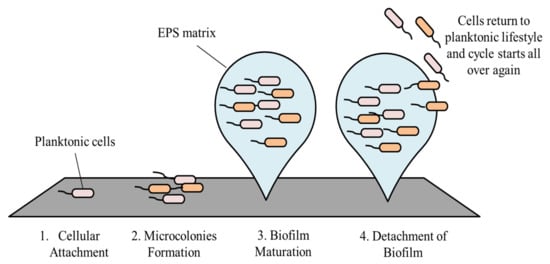
2. Stages in Biofilm Formation and Its Development
The development of biofilm involves four main stages, starting with cellular attachment, to formation of microcolonies, then biofilm maturation, and finally dispersion [
17].
2.1. Cellular Attachment
Attachment of bacteria onto a surface is the initial step of biofilm formation [
24]. In order for an organism to bind to a surface, they have to overcome the repulsive forces caused by the negatively charged bacterial membrane and the surface [
25]. A hydrophobic surface such as plastic has a reduced force of repulsion as compared to a hydrophilic surface, for example glass and metal. The reduction in repulsive forces corresponds to increased strength of attachment [
17]. Attachment is achieved by the presence of flagella and pili on the bacterial membrane [
17,
25]. The attachment phase involving the structural support is called a reversible attachment. The binding is reversible as bacteria are only poorly bound to a surface and are able to leave the surface at this stage. The bacteria leaving a surface return to their planktonic lifestyle [
26]. For bacteria that stays on the surface, they undergo irreversible attachment, a step that is important for the transition from a planktonic to biofilm lifestyle. The adhesion of cells to a surface is aided by the surface proteins of the bacterial cells, resulting in irreversible binding [
27]. As a result, the biofilm is now able to withstand stronger chemical and physical shear forces [
28].
2.2. Microcolonies Formation
After bacterial cells have attached irreversibly onto a surface, they start to divide and produce EPS [
1]. The production of EPS leads to the formation of a biofilm matrix, a ‘shelter’ where all the attached cells are living in [
29]. EPS is involved in the adhesion of cells to surfaces, contributing to permanent attachment [
27]. Recently, it was found that matrix proteins of a biofilm possess adhesin-like properties that mediate cellular attachment. Drescher et al. [
30] showed that RbmA, one of the matrix proteins found in the
Vibrio cholerae biofilm, acts as a mediator in this process. In addition to that, EPS mediates cellular cohesion in which bacterial cells are brought together and form microcolonies, the second step of biofilm formation [
31]. Usually, a biofilm contains more than one type of micro-community and involves coordination between one community to another. This coordination is important for substrate exchange, the distribution of essential metabolic products, and also the excretion of harmful substances [
17].
2.3. Biofilm Maturation
Cellular division with continuous production of EPS leads to the formation of an early biofilm, which becomes mature after some time and ultimately becomes a three-dimensional (3-D) structure. The aforementioned 3-D structure is contributed to by the EPS (produced by the embedded cells), and they are also responsible for maintaining this architecture [
32]. The maturation process involves cell-to-cell communication, in which cells embedded in the biofilm release signaling molecules called auto-inducers to facilitate quorum sensing [
17,
33]. Cells that receive the signals and then express their genes coding for EPS. With the increased production of EPS, biofilms acquire the previously described 3-D structure [
17,
34]. Besides that, a mature biofilm also contains water channels in the matrix acting as a circulatory system. The functions of these channels include nutrient distribution and the removal of waste products [
35,
36].
2.4. Detachment of Biofilm
Following maturation, biofilms undergo a process known as dispersion. At this stage, some of the cells leave the biofilm and return to their planktonic lifestyle [
37]. As these cells return to their free-floating form, they are now able to attach onto a new surface, and the cycle starts all over again [
21]. Cells can either detach from the biofilm actively or passively. Passive dispersal of a biofilm is mediated by mechanical forces or external forces such as abrasion, fluid shear, and solid shear [
38,
39]. In active dispersal, it involves the upregulation and downregulation of genes. Several environmental triggers are found to be related to the dispersal of biofilms, which include nutrient starvation, insufficient oxygen supply, and changes in temperature [
40]. Under these conditions, genes responsible for flagella synthesis are upregulated and this provides the bacterial cells the ability to leave the biofilm [
41]. Besides that, production of dispersin B is also enhanced. This enzyme is present in the extracellular matrix and functionally acts to hydrolyze polysaccharides, resulting in EPS degradation. Increased secretion of dispersin B into the matrix negatively impacts biofilm formation and allows adherent cells to leave the biofilm easily [
41,
42].
Figure 2, as shown, summarizes the stages in biofilm formation.
Figure 2. Stages in biofilm formation.
3. Quorum Sensing in Biofilm Formation
The term quorum sensing (QS) was first described by W.C. Fuqua, denoting the cell–cell interaction of bacteria [
79]. His findings were based on research from Tomasz and Nealson (as early as 1965 and 1970, respectively) who reported the discovery of autoinducer activity in
Vibrio fischeri [
80]. QS is crucial in microbial communities as bacteria can collectively obtain information about their population density, coordinate population behavior, and perform gene expression in response to variations of the surrounding population density [
81]. QS is extremely beneficial for microorganisms as certain biological processes are much more expensive and unproductive for a single bacterial cell to carry out [
82,
83]. For instance, the execution of activities such as bioluminescence, virulence factor production, and biofilm formation require a higher population density of bacteria for maximal performance [
84].
Extracellular signaling molecules known as autoinducers (AIs) govern the communication among cells through their secretion, detection, and responses [
85]. The concentration of AIs is monitored by the population density of bacteria in the environment. The concentration of AIs is said to be directly proportional to the bacterial community density as the increment of bacterial population contributes to an elevation in AIs concentration [
86].
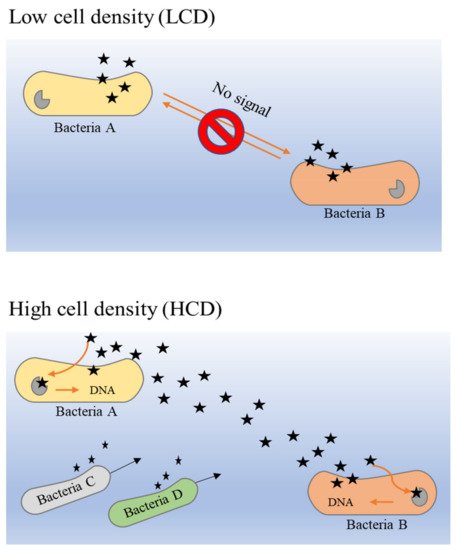
The principle of the QS system in bacteria is closely dependent on the release of AIs by bacteria in a particular community. The overall mechanism of AI release is different in low cell density (LCD) and high cell density (HCD) environments [
87]. As described previously, the larger the population density, the higher the concentration of secreted AIs. Hence, the concentration of AIs in HCD is comparatively higher than that in LCD, and the threshold of detection and response by receptors that are present in the bacterial membrane or cytoplasm can be attained easily. It is a circular loop denominated as a ‘feed-forward AIs loop’, which describes the detection of sufficient concentrations of AIs that enable bacteria to proceed into activation of gene expression alteration that could further promote more secretion of AI molecules [
88]. This loop is most probably the key to stimulating and maintaining synchrony in bacterial populations [
89].
From within bacterial cells, AHLs (acyl-homoserine lactones) and AIPs (auto-inducer peptides) are produced and processed before they are secreted into the extracellular space [
84]. When the extracellular concentration of AIs is elevated in HCD environments, these signaling molecules bind to the two-component histidine kinase receptor that is bound with the cognate membrane [
90,
91]. This activates a cascade reaction that has been activated initially through autophosphorylation to the cognate response regulator in the cytoplasm, then the activated regulator further activates transcription in the bacterial gene [
89].
Figure 3 illustrates the binding mechanism of AI molecules (bacterial QS) in LCD and HCD environments.
Figure 3. The binding mechanism of AIs molecules (bacterial QS) in LCD and HCD environments [
89,
90,
91].
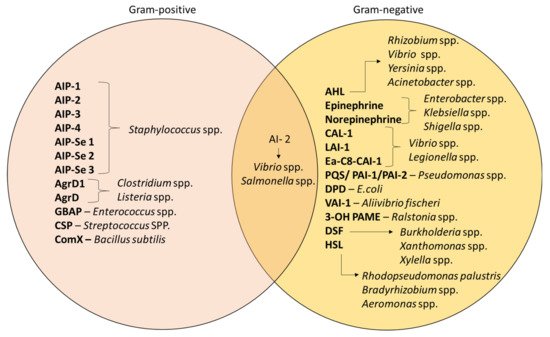
Despite QS being recognized as a general feature of bacteria, the mechanism still differs in terms of the signaling molecules involved; namely, AI-1 and AI-2. AI-1 molecules govern intraspecies communication and it involves the acyl-homoserine lactones (AHLs)-based interaction whereas AI-2 is produced by both type of bacteria, which is the furanosyl borate diester [
92]. Apart from that, studies have also differentiated the mechanism of AIs according to the types of bacteria [
93]. Gram-positive bacteria such as
Staphylococcus spp. and
Enterococcus spp. specifically synthesize AIPs for communication [
94].
Pseudomonas spp. and
Acinetobacter spp. are Gram-negative bacteria that produce AHLs as their signaling molecules; the composition of AHLs consist of a lactone ring with varying lengths of aliphatic acyl chain [
95]. In addition, these bacteria can also be controlled by other molecules that depend on S-adenosylmethionine as a substrate for their production [
89].
Figure 4 demonstrates the different types of AIs that are released by different bacteria [
84,
96]. For example,
Xanthomonas uses diffusible signal factor (DSF) [
97], whereas hydroxy palmitic acid methyl ester (3-OH PAME) is used by
Ralstonia spp. [
98].
Figure 4. Different types of AIs in Gram-positive and Gram-negative bacteria [
84,
92,
96,
97,
98].
4. Consequences of Biofilm Formation
4.1. Biofouling
In various industrial fields (including water, food, medical, shipping, and oil industries), one of the most common problem of biofilm formation is biofouling [
99,
100]. Biofouling (also known as biological fouling) is generally defined as the unwanted growth and agglomeration of living organisms on surfaces [
101]. Based on recent studies, it is now widely recognized that biofouling has negative impacts in many industries and can cost them their profitability [
102]. Biofouling in medical industries only involves biofilm formation, which contrasts with biofouling in marine, manufacturing industries, and food industries as it involves a combination of biofilm formation, macrofouling, and scaling [
103]. The physical and chemical properties of surfaces can influence the extent of bacterial adhesion [
104]. The morphology of biofouling can be distinguished based on the thickness, bioadhesive strength, density, and type of fouling organisms [
103]. For the most part, biofouling elicits a high cost in various industrial sectors due to its wide range of detrimental effects. The subsequent subsections review the aforesaid effects in more detail.
4.1.1. Biofouling in Marine Industries
Globally, more than four thousand marine species have been associated with causing biofouling, subsequently posing a more than a serious threat in marine parks and aquacultures [
105,
106,
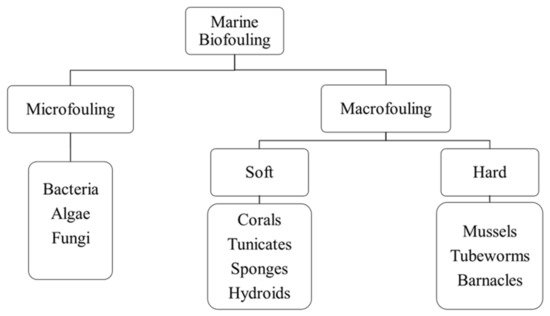
107]. Marine biofouling can be categorized as microfouling (which involves the accumulation of microorganisms) and macrofouling (which involves the accumulation of macro-organisms such as invertebrates), which can further be divided into soft and hard macrofouling [
104,
108]. Soft macrofouling invertebrates comprise corals, tunicates, sponges, and hydroids, whereas hard macrofouling invertebrates include mussels, tubeworms, and barnacles [
109,
110]. These marine fouling organisms dwell better in temperate and tropical environmental conditions, but this can fluctuate according to seasonal variations [
111]. According to Lebret et al. [
112], marine algae is predominant among these organisms as they colonize the fastest, adhere to a broad range of surfaces, and produce numerous metabolites that have antifungal and antimicrofouling properties. Notably, some studies have described microorganisms as being involved in biofouling forms microlayers, which then initiates the adhesion of macro-organisms that can subsequently develop into macrofouling [
100,
104,
109].
Figure 5 shows the classification of marine biofouling.
Figure 5. Classification of marine biofouling.
Marine biofouling appears as the visible aquatic growth of living organisms on seashore rocks, ships, boats, buoys, and underwater structures, which over time can incur physical stress on ship engines, stimulate biocorrosion of ship vessels, and interfere with mariculture environments [
103,
107]. A biofilm of 1 mm thickness is able to increase the friction drag of ship hulls by 80%, resulting in an overall 15% speed loss [
113]. Since biofouling creates surface roughness at ship hulls, this can cause elevated ship resistance, which increases the fuel consumption (with emission of greenhouse gases), leading to high maintenance costs. To reinforce this, a study conducted by Baciocco [
114] revealed that in the year 1974, the US Navy spent 200 million dollars to cover shipping maintenance and marine deterioration costs, both of which were elevated due to biofouling.
Biofouling can accelerate the decay of ship coatings due to the colonization of fouling marine species, further affecting the ship’s performance (causing unwanted noises, reduced speed, and an increase in fuel usage). This leads to an increment in unnecessary dry-docking procedures (a method of ship repair) and repainting, increasing the overall burden for more maintenance costs [
107,
111]. According to Munk et al. [
115], ship vessel owners spend millions of dollars every three to five years just to cover the costs for dry-docking procedures, which involves the replacement of the hull coating, the cleaning of hulls, and the polishing of propellers. The accumulation of biofouling algae communities on ship walkways and structures can create a slippery-like coating, which may pose a potential safety hazard if one is not carefully onboard [
103].
4.1.2. Biofouling in Food and Beverage Industries
In the food industry, biofouling is prominent in processing appliances and piping systems, which gives rise to contamination, poor hygiene standards, corrosion of food equipment, food spoilage, decreased shelf-life of food products, and foodborne diseases [
102,
116,
117]. This in turn generates functional and hygienic complications with pronounced financial losses and risks to human health [
118]. These foodborne illnesses are a high risk for those who mostly consume raw food or ready-to-eat (RTE) products [
119]. The severity of the diseases can range from mild gastroenteritis to life-threatening complications including liver abnormalities, meningitis, thrombotic thrombocytopenic purpura, and many others. The various environmental parameters as well as the EPS favors the persistence of biofilms in food industries, causing the overall deterioration of biofouling-susceptible appliances (pipelines, tanks, packing tools, storage materials, dispensing tubes, and pasteurizer plates) that are made of metals and alloys [
120]. Sixty percent of foodborne infections were due to biofilm transfer from food-processing equipment to processed food items (which are then consumed by unsuspecting individuals) [
121]. Furthermore, these surface-attached microorganisms cannot be easily eliminated as they are highly resistant to antimicrobials, ultimately contributing to severe health risks [
102]. Among the various bacteria that form biofilms,
Escherichia coli has been reported as the most tenacious foodborne pathogen commonly found in meat industries, vegetable processing industries, and preserved products [
118]. Other notable bacteria that form resilient biofilms on food surfaces and are potential foodborne pathogens include
L. monocytogenes,
S. enterica,
C. jejuni,
C. coli,
S. typhimurium,
S. enteritidis,
B. cereus, and
Pseudomonas spp. [
118,
119,
120,
121,
122,
123,
124,
125,
126,
127,
128,
129,
130,
131,
132].
Table 2 summarizes the various foodborne infections caused by biofilms in different types of food industry.
Table 2. Effects of biofouling in food and beverage industries.
| Type of Food Industry |
Prominent Bacteria |
Effects |
References |
| Dairy Industry |
L. monocytogenes
S. typhimurium and S. enteritidis
E. coli (STEC)
B. cereus |
Gastroenteritis or listeriosis
Gastroenteritis
Enterohemorrhagic gastroenteritis or hemolytic uremic syndrome (HUS)
Gastroenteritis or occasionally acute liver failure |
[120,122,126,129,131] |
| Poultry Industry |
S. enterica
C. jejuni and C. coli |
Gastroenteritis or septicemia
Enterocolitis or gastroenteritis |
[120,123,128] |
| Meat Industry |
E. coli O157:H7
L. Monocytogenes
Salmonella spp. |
Hemorrhagic colitis or thrombotic thrombocytopenic purpura (TTP)
Gastroenteritis or listeriosis
Salmonellosis |
[118,124,127,130,132] |
| Fish and Seafood Industry |
Vibrio cholerae
Aeromonas spp.
Pseudomonas spp. |
Cholera or gastroenteritis
Epizootic ulcerative syndrome (EUS) |
[119,121,122,125] |
4.1.3. Biofouling in Medical Industries
In medical industries, biofouling can occur in medical devices including urinary catheters, contact lenses, prosthetic implants, breast implants, dental implants, tissue fillers, cerebrospinal fluid shunts, and biosensors. As the use of these medical devices increases, the risk of biofilm infections has also increased [
133]. Most of these medical devices are made of polymers, ceramics, and composites materials [
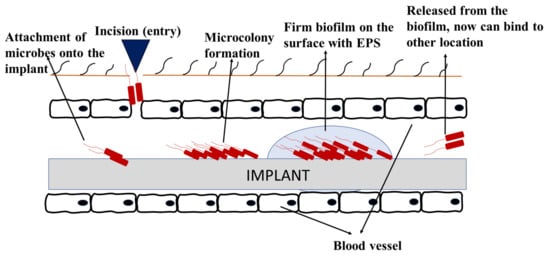
18]. Biofouling in implanted devices initiates soon after the insertion of device, whereby the host-derived adhesins form a conditional layer and attracts the planktonic cells to attach onto the implant surface. Following the stages on how biofilm forms, signaling occurs, and the bacteria persists [
134].
Figure 6 shows an example of how biofouling can develop on implanted medical devices. Biofilms on medical devices can arise from the patient’s skin, healthcare workers, or the surrounding environment [
135]. This can cause various detrimental effects of medical biofouling, especially implant-related diseases, malfunction of devices, and implant rejection [
103,
136].
Figure 6. Biofouling on medical implant devices.
The malfunction of medical implanted devices results in costly surgical removal and replacement procedures [
137,
138]. According to Bixler and Bhushan [
103], approximately 45% of nosocomial infections are attributed to implant-related diseases with over 5000 deaths per annum. The mortality rates associated with these diseases are especially high for those with contaminated cardiovascular implants i.e., prosthetic heart valves and aortic grafts [
134]. In a US survey, results showed that almost 25% of blood infection-related mortality resulted from implanted vascular catheters [
135]. For the most part, surgical removal is required for those implants infected by different organisms [
134]. In fact, approximately two-thirds of medical implant-related infections are a result of
S. aureus or the coagulase negative
Staphylococci [
134].
Apart from that, both the Gram-positive and Gram-negative bacteria can adhere onto medical devices and form biofilms. Some notable and well-known biofilm-forming bacteria that can cause medical implant infections include
E. coli,
P. aeruginosa,
C. albicans,
K. pneumoniae,
S. aureus, and
S. epidermidis [
18,
103,
135]. An adherent biofilm can develop and lead to acute fungemia. Several studies have shown that the cells detach from a biofilm are related to mortality and cytotoxicity. Infections caused by
Candida spp. are very common in devices like catheters.
Aspergillus spp. are commonly attributed to infections involving cardiac pacemakers, joint replacements, breast implants, and cardiac valves.
Cryptococcus neoformans can also colonize devices similar to the aforementioned examples and cause severe infections [
139].
4.2. Biofilm-Related Infections
Approximately 65% of infections that are caused by bacteria are associated with biofilms [
17]. Some of the common non-device-related biofilm infections are discussed in the following subsections.
4.2.1. Periodontitis
Periodontitis is an infection of the gums whereby the soft tissues and the bones supporting the teeth gets damaged as a result of the infection. This could develop due to poor oral hygiene, which may also result in tooth loss. The infectious agents behind this infection are
Pseudomonas aerobicus and
Fusobacterium nucleatum [
17]. These bacteria can form biofilms in the mucosal surfaces of the mouth. Through this way, they can invade the cells, release their toxins, and form plaques in a couple of weeks [
17]. Moreover, the formation of lesions may be present due to the infection. The result of the treatment may be influenced by the size of the lesion [
140]. Antimicrobial treatment is enough in the case of minor infections, but special treatment may be required when the infection becomes severe [
141].
4.2.2. Rhinosinusitis
The inflammation of the paranasal sinuses is referred to as sinusitis or rhinosinusitis. Depending on the severity of the infection, it can be classified as acute or chronic. It is said that most of this infection is caused by the colonization of bacteria. However, some studies have also stated that fungal biofilms might also be involved. Both
Aspergillus fumigatus and
Staphylococcus aureus have been discovered to be involved in causing rhinosinusitis [
142]. It has also been stated that the patients that have undergone surgical procedures are more likely to be infected with the infection related to biofilms. Biofilms are formed in the presence of more eosinophil cells. Moreover, bacteria such as
P. aeruginosa,
S. pneumoniae,
S. aureus, and
H. influenza are also involved in causing chronic rhinosinusitis [
143]. The most dominant species is
S. aureus, which was proven in a study conducted by Schurmann et al. [
144]. It was further concluded that most of the chronic rhinosinusitis infections are associated with microbiomes comprising of multiple different species.
4.2.3. Cystic Fibrosis (CF)
Cystic fibrosis is an autosomal recessive disease caused by a mutation in the gene coding for the cystic fibrosis transmembrane conductance regulator (CFTR) protein. The most affected areas are the gut and pancreas in which there is a failure to remove the mucous secretions. It mainly involves constant coughing, and the lungs tend to be more prone to infections [
145]. Almost 80% of infections seen in cystic fibrosis are related to biofilms. The lung environment of a CF patient is favorable to
P. aeruginosa and that is why the bacterium is able to dominate in the airways. Their resistance to antibiotics occurs due to increased production of EPS [
146]. Studies have shown that the presence of the microbial agent leads to poor prognosis in CF patients.
P. aeruginosa is able to form drug-resistant biofilms, which makes antibacterial therapy useless [
147,
148].
This entry is adapted from the peer-reviewed paper 10.3390/medicina57080839