Interferons (IFNs) are cytokines involved in the immune response that act on innate and adaptive immunity. These proteins are natural cell-signaling glycoproteins expressed in response to viral infections, tumors, and biological inducers and constitute the first line of defense of vertebrates against infectious agents. They have been used in different presentations for several therapy applications. However, their administration has presented difficulties due to the molecules’ size, sensitivity to degradation, and rapid elimination from the bloodstream. An alternative to overcome these drawbacks is to formulate drug delivery systems to provide adequate therapeutic concentrations for these cytokines, decrease their toxicity and prolong their half-life in the circulation.
- interferons
- antiviral
- antiproliferative
- immunomodulator
- PEGylation
- drug delivery system
- liposomes
- polymeric micelles
- microparticles
- nanoparticles
1. Introduction
2. IFN Delivery Systems
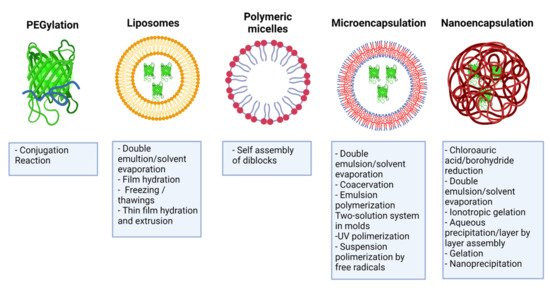
3. PEGylation of IFNs
4. Liposomes
Liposomes are spherical structures formed by one or more concentric lipid bilayers surrounding aqueous spaces [53]. They consist of phospholipids and cholesterol, formed by hydrophobic interactions and other intermolecular forces, and possess hydrophobic and hydrophilic regions [54]. Liposome-based drug delivery systems have shown unique characteristics to cross biological obstacles and improve pharmacodynamics [55]. Some of the advantages of this delivery system include biocompatibility, low immunogenicity, self-assembly ability, and the ability to transport drugs, such as IFNs, thereby reducing systemic toxicity and prolonging residence time in the circulation [53]. There are different liposomal formulations for encapsulating chemotherapeutic drugs, antifungals, and vaccines, currently approved by regulatory agencies for clinical application [56].
Gurari-Rotman and Lelkes reported the first encapsulation of IFN-α in multivesicular liposomes in 1982 [57]. Consequently, similar investigations were developed with IFN-γ [58][59]. New formulations were developed at the beginning of the 21st century, using different strategies to improve encapsulation efficiency.
5. Polymeric Micelles
Polymeric micelles are nanocarriers formed by the spontaneous arrangement of amphiphilic block copolymers in aqueous solutions [60]. Block copolymers are macromolecules of two or more different polymers joined by covalent bonds to form one structure. Its molecular conformation depends upon the number of blocks. Diblock copolymer consists of two homopolymers, while triblock copolymer has three homopolymers. More complicated architectures such as mixed arm block copolymers contain three polymer chains covalently joined at a common branching point [61]. Polymeric micelles possess a two-phase structure: a hydrophobic core and a hydrophilic corona that allows modifications to their surface [30]. Polymeric micelles have several advantages for drug delivery, such as their increased solubility, enhanced stability of the molecule, structural flexibility, capacity to encapsulate a wide range of therapeutics, and the possibility of adjusting their size at the nanometer scale [30][61][62]. Modifications in the corona make it possible to reduce their clearance by the RES, thus prolonging their circulation time [63]. In this way, it is feasible to decrease the drug dose and the toxicity associated with drugs such as IFNs [30].
6. Recent Encapsulation Forms of IFNs
6.1. Microencapsulation
6.2. Nanoencapsulation
7. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13101533
References
- Negishi, H.; Taniguchi, T.; Yanai, H. The Interferon (IFN) Class of Cytokines and the IFN Regulatory Factor (IRF) Transcription Factor Family. Cold Spring Harb. Perspect. Biol. 2018, 10, a028423.
- Li, S.-F.; Zhao, F.-R.; Shao, J.-J.; Xie, Y.-L.; Chang, H.-Y.; Zhang, Y.-G. Interferon-omega: Current status in clinical applications. Int. Immunopharmacol. 2017, 52, 253–260.
- Blank, T.; Prinz, M. Type I interferon pathway in CNS homeostasis and neurological disorders. Glia 2017, 65, 1397–1406.
- Schreiber, G. The molecular basis for differential type I interferon signaling. J. Biol. Chem. 2017, 292, 7285–7294.
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584.
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923.
- Wang, B.X.; Fish, E.N. The yin and yang of viruses and interferons. Trends Immunol. 2012, 33, 190–197.
- Dickow, J.; Francois, S.; Kaiserling, R.-L.; Malyshkina, A.; Drexler, I.; Westendorf, A.M.; Lang, K.S.; Santiago, M.L.; Dittmer, U.; Sutter, K. Diverse Immunomodulatory Effects of Individual IFN-α Subtypes on Virus-Specific CD8+ T Cell Responses. Front. Immunol. 2019, 10, 2255.
- Benedicenti, O.; Wang, T.; Morel, E.; Secombes, C.J.; Soleto, I.; Díaz-Rosales, P.; Tafalla, C. Type I Interferon Regulates the Survival and Functionality of B Cells in Rainbow Trout. Front. Immunol. 2020, 11, 1494.
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061, doi:10.3389/fimmu .2018.02061.
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510, doi:10.1038/ni1582.
- Goldszmid, R.S.; Caspar, P.; Rivollier, A.; White, S.; Dzutsev, A.; Hieny, S.; Kelsall, B.; Trinchieri, G.; Sher, A. NK Cell-Derived Interferon-γ Orchestrates Cellular Dynamics and the Differentiation of Monocytes into Dendritic Cells at the Site of Infection. Immunity 2012, 36, 1047–1059, doi:10.1016/j.immuni.2012.03.026.
- Malone, R.M.; Cox, B.C.; Frogget, B.C.; Kaufman, M.I.; Tibbitts, A.; Tunnell, T.W.; Evans, S.C.; Herrmann, H.W.; Kim, Y.H.; Mack, J.M. Overview of the gamma reaction history diagnostic for the National Ignition Facility (NIF). In Proceedings of the International Optical Design Conference, Jackson Hole, WY, USA, 13–17 June 2010; p. ITuC3.
- Kaskow, B.J.; Baecher-Allan, C. Effector T Cells in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a029025, doi:10.1101/cshperspect.a029025.
- Green, D.S.; Young, H.A.; Valencia, J.C. Current prospects of type II interferon γ signaling and autoimmunity. J. Biol. Chem. 2017, 292, 13925–13933, doi:10.1074/jbc.r116.774745.
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodriguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15.
- Kozlowski, A.; Harris, J.M. Improvements in protein PEGylation: Pegylated interferons for treatment of hepatitis C. J. Control. Release 2001, 72, 217–224.
- Chen, J.; Guo, Z.; Tian, H.; Chen, X. Production and clinical development of nanoparticles for gene delivery. Mol. Ther. Methods Clin. Dev. 2016, 3, 16023.
- Cocco, E.; Marrosu, M.G. Profile of PEGylated interferon beta in the treatment of relapsing-remitting multiple sclerosis. Ther. Clin. Risk Manag. 2015, 11, 759–766.
- Beilharz, M.W.; Cummins, M.J.; Bennett, A.L.; Cummins, J.M. Oromucosal Administration of Interferon to Humans. Pharmaceuticals 2010, 3, 323–344.
- Jakimovski, D.; Kolb, C.; Ramanathan, M.; Zivadinov, R.; Weinstock-Guttman, B. Interferon β for Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a032003.
- Castro, F.; Cardoso, A.P.; Goncalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847.
- Conlon, K.C.; Miljković, M.D.; Waldmann, T.A. Cytokines in the Treatment of Cancer. J. Interf. Cytokine Res. 2019, 39, 6–21.
- Fagundes, R.N.; Ferreira, L.; Pace, F.H.L. Health-related quality of life and fatigue in patients with chronic hepatitis C with therapy with direct-acting antivirals agents interferon-free. PLoS ONE 2020, 15, e0237005.
- Park, A.; Iwasaki, A. Type I and Type III Interferons—Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe 2020, 27, 870–878.
- Pandey, A. Solid lipid nanoparticles: A multidimensional drug delivery system. In Nanoscience in Medicine Vol. 1; Springer: Berlin/Heidelberg, Germany, 2020; pp. 249–295.
- Ye, C.; Chi, H. A review of recent progress in drug and protein encapsulation: Approaches, applications and challenges. Mater. Sci. Eng. C 2018, 83, 233–246.
- Sánchez, A.; Tobío, M.; González, L.; Fabra, A.; Alonso, M.J. Biodegradable micro- and nanoparticles as long-term delivery vehicles for interferon-alpha. Eur. J. Pharm. Sci. 2003, 18, 221–229.
- Dai, J.; Long, W.; Liang, Z.; Wen, L.; Yang, F.; Chen, G. A novel vehicle for local protein delivery to the inner ear: Injectable and biodegradable thermosensitive hydrogel loaded with PLGA nanoparticles. Drug Dev. Ind. Pharm. 2018, 44, 89–98.
- Majumder, N.; Das, N.G.; Das, S.K. Polymeric micelles for anticancer drug delivery. Ther. Deliv. 2020, 11, 613–635.
- Liu, X.; Sun, M.; Sun, J.; Hu, J.; Wang, Z.; Guo, J.; Gao, W. Polymerization Induced Self-Assembly of a Site-Specific Interferon α-Block Copolymer Conjugate into Micelles with Remarkably Enhanced Pharmacology. J. Am. Chem. Soc. 2018, 140, 10435–10438.
- Wang, Z.; Guo, J.; Liu, X.; Sun, J.; Gao, W. Temperature-triggered micellization of interferon alpha-diblock copolypeptide conjugate with enhanced stability and pharmacology. J. Control. Release 2020, 328, 444–453.
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control. Release 2016, 224, 86–102.
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656.
- Lembo, D.; Donalisio, M.; Civra, A.; Argenziano, M.; Cavalli, R. Nanomedicine formulations for the delivery of antiviral drugs: A promising solution for the treatment of viral infections. Expert Opin. Drug Deliv. 2018, 15, 93–114.
- Thitinan, S.; McConville, J.T. Interferon alpha delivery systems for the treatment of hepatitis C. Int. J. Pharm. 2009, 369, 121–135.
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J. Pharm. Sci. 2016, 105, 460–475.
- Delgado, C.; Francis, G.E.; Fisher, D. The uses and properties of PEG-linked proteins. Crit. Rev. Ther. Drug Carr. Syst. 1992, 9, 249–304.
- Nucci, M.L.; Shorr, R.; Abuchowski, A. The therapeutic value of poly(ethylene glycol)-modified proteins. Adv. Drug Deliv. Rev. 1991, 6, 133–151.
- Foster, G.R. Pegylated Interferons for the Treatment of Chronic Hepatitis C. Drugs 2010, 70, 147–165, doi:10.2165/11531990-000000000-00000.
- Veronese, F.M.; Mero, A. The Impact of PEGylation on Biological Therapies. BioDrugs 2008, 22, 315–329, doi:10.2165/00063030-200822050-00004.
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J. Pharm. Sci. 2016, 105, 460–475, doi:10.1016/j.xphs.2015.11.015.
- Asselah, T.; Lada, O.; Moucari, R.; Martinot, M.; Boyer, N.; Marcellin, P. Interferon Therapy for Chronic Hepatitis B. Clin. Liver Dis. 2007, 11, 839–849.
- Trinh, V.A.; Zobniw, C.; Hwu, W.-J. The efficacy and safety of adjuvant interferon-alfa therapy in the evolving treatment landscape for resected high-risk melanoma. Expert Opin. Drug Saf. 2017, 16, 933–940.
- Abdolvahab, M.H.; Mofrad, M.; Schellekens, H. Interferon Beta: From Molecular Level to Therapeutic Effects. Int. Rev. Cell Mol. Biol. 2016, 326, 343–372.
- Jansen, P.L.; De Bruijne, J. Controlled-release interferon alpha 2b, a new member of the interferon family for the treatment of chronic hepatitis C. Expert Opin. Investig. Drugs 2012, 21, 111–118.
- Maughan, A.; Ogbuagu, O. Pegylated interferon alpha 2a for the treatment of hepatitis C virus infection. Expert Opin. Drug Metab. Toxicol. 2018, 14, 219–227.
- Woo, A.S.J.; Kwok, R.; Ahmed, T. Alpha-interferon treatment in hepatitis B. Ann. Transl. Med. 2017, 5, 159.
- Boulestin, A.; Kamar, N.; Sandres-Saune, K.; Alric, L.; Vinel, J.-P.; Rostaing, L.; Izopet, J. Pegylation of IFN-α and Antiviral Activity. J. Interf. Cytokine Res. 2006, 26, 849–853.
- Meller, S.; Gerber, P.; Kislat, A.; Hevezi, P.; Göbel, T.; Wiesner, U.; Kellermann, S.; Bünemann, E.; Zlotnik, A.; Häussinger, D. Allergic sensitization to pegylated interferon-α results in drug eruptions. Allergy 2015, 70, 775–783.
- Wang, G.; Hu, J.; Gao, W. Tuning the molecular size of site-specific interferon-polymer conjugate for optimized antitumor efficacy. Sci. China Mater. 2017, 60, 563–570.
- Bewersdorf, J.P.; Giri, S.; Wang, R.; Podoltsev, N.; Williams, R.T.; Rampal, R.K.; Tallman, M.S.; Zeidan, A.M.; Stahl, M. Interferon Therapy in Myelofibrosis: Systematic Review and Meta-analysis. Clin. Lymphoma Myeloma Leuk. 2020, 20, e712–e723.
- Vahed, S.Z.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-based drug co-delivery systems in cancer cells. Mater. Sci. Eng. C 2017, 71, 1327–1341.
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851.
- Zahednezhad, F.; Saadat, M.; Valizadeh, H.; Zakeri-Milani, P.; Baradaran, B. Liposome and immune system interplay: Challenges and potentials. J. Control. Release 2019, 305, 194–209.
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29.
- Gurari-Rotman, D.; Lelkes, P.I. Encapsulation of human fibroblast interferon activity in liposomes. Biochem. Biophys. Res. Commun. 1982, 107, 136–143.
- Eppstein, D.A.; Marsh, Y.V.; van der Pas, M.; Felgner, P.L.; Schreiber, A.B. Biological activity of liposome-encapsulated murine interferon gamma is mediated by a cell membrane receptor. Proc. Natl. Acad. Sci. USA 1985, 82, 3688–3692.
- Sone, S.; Tandon, P.; Utsugi, T.; Ogawara, M.; Shimizu, E.; Nii, A.; Ogura, T. Synergism of recombinant human interferon gamma with liposome-encapsulated muramyl tripeptide in activation of the tumoricidal properties of human monocytes. Int. J. Cancer 1986, 38, 495–500.
- Amin, M.C.I.M.; Butt, A.M.; Amjad, M.W.; Kesharwani, P. Chapter 5—Polymeric Micelles for Drug Targeting and Delivery. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Mishra, V., Kesharwani, P., Mohd Amin, M.C.I., Iyer, A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 167–202.
- Bastakoti, B.P.; Liu, Z. Chapter 10—Multifunctional polymeric micelles as therapeutic nanostructures: Targeting, imaging, and triggered release. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 261–283.
- Liu, J.; Ai, X.; Zhang, H.; Zhuo, W.; Mi, P. Polymeric Micelles with Endosome Escape and Redox-Responsive Functions for Enhanced Intracellular Drug Delivery. J. Biomed. Nanotechnol. 2019, 15, 373–381.
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 691–707.
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340.
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197.
- Yasukawa, T.; Ogura, Y.; Tabata, Y.; Kimura, H.; Wiedemann, P.; Honda, Y. Drug delivery systems for vitreoretinal diseases. Prog. Retin. Eye Res. 2004, 23, 253–281.
- Saez, V.; Ramon, J.; Peniche, C.; Hardy, E. Microencapsulation of Alpha Interferons in Biodegradable Microspheres. J. Interf. Cytokine Res. 2012, 32, 299–311.
- Lembo, D.; Cavalli, R. Nanoparticulate Delivery Systems for Antiviral Drugs. Antivir. Chem. Chemother. 2010, 21, 53–70.
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162.
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524–539.
- Castro, L.S.; Lobo, G.S.; Pereira, P.; Freire, M.G.; Neves, M.C.; Pedro, A.Q. Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation. Vaccines 2021, 9, 328.
