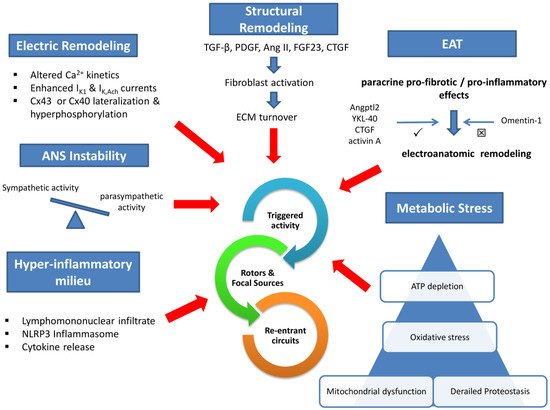
Electrical remodeling lies on impaired calcium handling, enhanced inwardly rectifying potassium currents, and gap junction perturbations. In addition, a wide array of profibrotic stimuli activates fibroblast to an increased extracellular matrix turnover via various intermediaries. Concomitant dysregulation of the autonomic nervous system and the humoral function of increased epicardial adipose tissue (EAT) are established mediators in the pathophysiology of AF. Local atrial lymphomononuclear cells infiltrate and increased inflammasome activity accelerate and perpetuate arrhythmia substrate. Finally, impaired intracellular protein metabolism, excessive oxidative stress, and mitochondrial dysfunction deplete atrial cardiomyocyte ATP and promote arrhythmogenesis.
- atrial fibrillation
- therapeutic implications
- pathogenesis
1. Introduction
2. Atrial Fibrillation Pathogenesis
2.1. Mechanistic Approach
2.2. Molecular Pathophysiology
2.2.1. Ionic Perturbations
2.2.2. Structural Changes
2.2.3. Epicardial Adipose Tissue (EAT) and Autonomic Nervous System (ANS)
2.2.4. The Role of Inflammation
2.2.5. The Role of Proteostasis, Oxidative Stress, and Mitochondrial Bioenergetics
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics11091584
References
- Hobbelt, A.H.; Siland, J.E.; Geelhoed, B.; Van Der Harst, P.; Hillege, H.L.; Van Gelder, I.C.; Rienstra, M. Clinical, biomarker, and genetic predictors of specific types of atrial fibrillation in a community-based cohort: Data of the PREVEND study. EP Eur. 2017, 19, 226–232.
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century. Circ. Res. 2020, 127, 4–20.
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498.
- Khasnis, A.; Thakur, R.K. Atrial Fibrillation: A Historical Perspective. Cardiol. Clin. 2009, 27, 1–12.
- Perino, A.C.; Leef, G.C.; Cluckey, A.; Yunus, F.N.; Askari, M.; Heidenreich, P.A.; Narayan, S.M.; Wang, P.J.; Turakhia, M.P. Secular trends in success rate of catheter ablation for atrial fibrillation: The SMASH-AF cohort. Am. Heart J. 2019, 208, 110–119.
- Burashnikov, A. Investigational Anti–Atrial Fibrillation Pharmacology and Mechanisms by Which Antiarrhythmics Terminate the Arrhythmia: Where Are We in 2020? J. Cardiovasc. Pharmacol. 2020, 76, 492–505.
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666.
- Lalani, G.G.; Schricker, A.; Gibson, M.; Rostamian, A.; Krummen, D.E.; Narayan, S.M. Atrial Conduction Slows Immediately Before the Onset of Human Atrial Fibrillation. J. Am. Coll. Cardiol. 2012, 59, 595–606.
- Narayan, S.M.; Franz, M.R.; Clopton, P.; Pruvot, E.J.; Krummen, D.E. Repolarization Alternans Reveals Vulnerability to Human Atrial Fibrillation. Circulation 2011, 123, 2922–2930.
- Narayan, S.M.; Baykaner, T.; Clopton, P.; Schricker, A.; Lalani, G.G.; Krummen, D.E.; Shivkumar, K.; Miller, J.M. Ablation of Rotor and Focal Sources Reduces Late Recurrence of Atrial Fibrillation Compared with Trigger Ablation Alone. J. Am. Coll. Cardiol. 2014, 63, 1761–1768.
- Atienza, F.; Almendral, J.; Ormaetxe, J.M.; Moya, Á.; Martínez-Alday, J.D.; Hernández-Madrid, A.; Castellanos, E.; Arribas, F.; Arias, M.Á.; Tercedor, L.; et al. Comparison of Radiofrequency Catheter Ablation of Drivers and Circumferential Pulmonary Vein Isolation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2014, 64, 2455–2467.
- Voigt, N.; Heijman, J.; Wang, Q.; Chiang, D.Y.; Li, N.; Karck, M.; Wehrens, X.H.T.; Nattel, S.; Dobrev, D. Cellular and Molecular Mechanisms of Atrial Arrhythmogenesis in Patients with Paroxysmal Atrial Fibrillation. Circulation 2014, 129, 145–156.
- Vest, J.A.; Wehrens, X.H.T.; Reiken, S.R.; Lehnart, S.E.; Dobrev, D.; Chandra, P.; Danilo, P.; Ravens, U.; Rosen, M.R.; Marks, A.R. Defective Cardiac Ryanodine Receptor Regulation During Atrial Fibrillation. Circulation 2005, 111, 2025–2032.
- Voigt, N.; Li, N.; Wang, Q.; Wang, W.; Trafford, A.W.; Abu-Taha, I.; Sun, Q.; Wieland, T.; Ravens, U.; Nattel, S.; et al. Enhanced Sarcoplasmic Reticulum Ca2+ Leak and Increased Na+−Ca2+ Exchanger Function Underlie Delayed Afterdepolarizations in Patients with Chronic Atrial Fibrillation. Circulation 2012, 125, 2059–2070.
- Neef, S.; Dybkova, N.; Sossalla, S.; Ort, K.R.; Fluschnik, N.; Neumann, K.; Seipelt, R.; Schöndube, F.A.; Hasenfuss, G.; Maier, L.S. CaMKII-Dependent Diastolic SR Ca2+ Leak and Elevated Diastolic Ca2+ Levels in Right Atrial Myocardium of Patients with Atrial Fibrillation. Circ. Res. 2010, 106, 1134–1144.
- Yan, J.; Zhao, W.; Thomson, J.K.; Gao, X.; DeMarco, D.M.; Carrillo, E.; Chen, B.; Wu, X.; Ginsburg, K.S.; Bakhos, M.; et al. Stress Signaling JNK2 Crosstalk with CaMKII Underlies Enhanced Atrial Arrhythmogenesis. Circ. Res. 2018, 122, 821–835.
- Campbell, H.M.; Quick, A.P.; Abu-Taha, I.; Chiang, D.Y.; Kramm, C.F.; Word, T.A.; Brandenburg, S.; Hulsurkar, M.; Alsina, K.M.; Liu, H.-B.; et al. Loss of SPEG Inhibitory Phosphorylation of Ryanodine Receptor Type-2 Promotes Atrial Fibrillation. Circulation 2020, 142, 1159–1172.
- Herraiz-Martínez, A.; Tarifa, C.; Jiménez-Sábado, V.; Llach, A.; Godoy-Marín, H.; Colino-Lage, H.; Nolla-Colomer, C.; Casabella-Ramon, S.; Izquierdo-Castro, P.; Benítez, I.; et al. Influence of sex on intracellular calcium homoeostasis in patients with atrial fibrillation. Cardiovasc. Res. 2021.
- Christ, T.; Boknik, P.; Wöhrl, S.; Wettwer, E.; Graf, E.M.; Bosch, R.F.; Knaut, M.; Schmitz, W.; Ravens, U.; Dobrev, D. L-Type Ca2+ Current Downregulation in Chronic Human Atrial Fibrillation Is Associated With Increased Activity of Protein Phosphatases. Circulation 2004, 110, 2651–2657.
- Barana, A.; Matamoros, M.; Dolz-Gaitón, P.; Pérez-Hernández, M.; Amorós, I.; Núñez, M.; Sacristán, S.; Pedraz, Á.; Pinto, Á.; Fernández-Avilés, F.; et al. Chronic Atrial Fibrillation Increases MicroRNA-21 in Human Atrial Myocytes Decreasing L-Type Calcium Current. Circ. Arrhythmia Electrophysiol. 2014, 7, 861–868.
- Lu, Y.; Zhang, Y.; Wang, N.; Pan, Z.; Gao, X.; Zhang, F.; Zhang, Y.; Shan, H.; Luo, X.; Bai, Y.; et al. MicroRNA-328 Contributes to Adverse Electrical Remodeling in Atrial Fibrillation. Circulation 2010, 122, 2378–2387.
- Kettlewell, S.; Saxena, P.; Dempster, J.; Colman, M.A.; Myles, R.C.; Smith, G.L.; Workman, A.J. Dynamic clamping human and rabbit atrial calcium current: Narrowing I CaL window abolishes early afterdepolarizations. J. Physiol. 2019, 597, 3619–3638.
- Qi, X.-Y.; Vahdati Hassani, F.; Hoffmann, D.; Xiao, J.; Xiong, F.; Villeneuve, L.R.; Ljubojevic-Holzer, S.; Kamler, M.; Abu-Taha, I.; Heijman, J.; et al. Inositol Trisphosphate Receptors and Nuclear Calcium in Atrial Fibrillation. Circ. Res. 2021, 128, 619–635.
- Dobrev, D.; Friedrich, A.; Voigt, N.; Jost, N.; Wettwer, E.; Christ, T.; Knaut, M.; Ravens, U. The G Protein–Gated Potassium Current IK,ACh Is Constitutively Active in Patients With Chronic Atrial Fibrillation. Circulation 2005, 112, 3697–3706.
- Biliczki, P.; Boon, R.A.; Girmatsion, Z.; Bukowska, A.; Ördög, B.; Kaess, B.M.; Hohnloser, S.H.; Goette, A.; Varró, A.; Moritz, A.; et al. Age-related regulation and region-specific distribution of ion channel subunits promoting atrial fibrillation in human left and right atria. EP Eur. 2019, 21, 1261–1269.
- Luo, X.; Pan, Z.; Shan, H.; Xiao, J.; Sun, X.; Wang, N.; Lin, H.; Xiao, L.; Maguy, A.; Qi, X.-Y.; et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J. Clin. Investig. 2013, 123, 1939–1951.
- Girmatsion, Z.; Biliczki, P.; Bonauer, A.; Wimmer-Greinecker, G.; Scherer, M.; Moritz, A.; Bukowska, A.; Goette, A.; Nattel, S.; Hohnloser, S.H.; et al. Changes in microRNA-1 expression and IK1 up-regulation in human atrial fibrillation. Heart Rhythm 2009, 6, 1802–1809.
- Voigt, N.; Trausch, A.; Knaut, M.; Matschke, K.; Varró, A.; Van Wagoner, D.R.; Nattel, S.; Ravens, U.; Dobrev, D. Left-to-Right Atrial Inward Rectifier Potassium Current Gradients in Patients with Paroxysmal Versus Chronic Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2010, 3, 472–480.
- Andelova, K.; Egan Benova, T.; Szeiffova Bacova, B.; Sykora, M.; Prado, N.J.; Diez, E.R.; Hlivak, P.; Tribulova, N. Cardiac Connexin-43 Hemichannels and Pannexin1 Channels: Provocative Antiarrhythmic Targets. Int. J. Mol. Sci. 2020, 22, 260.
- Adam, O.; Lavall, D.; Theobald, K.; Hohl, M.; Grube, M.; Ameling, S.; Sussman, M.A.; Rosenkranz, S.; Kroemer, H.K.; Schäfers, H.-J.; et al. Rac1-Induced Connective Tissue Growth Factor Regulates Connexin 43 and N-Cadherin Expression in Atrial Fibrillation. J. Am. Coll. Cardiol. 2010, 55, 469–480.
- Dai, W.; Chao, X.; Li, S.; Zhou, S.; Zhong, G.; Jiang, Z. Long Noncoding RNA HOTAIR Functions as a Competitive Endogenous RNA to Regulate Connexin43 Remodeling in Atrial Fibrillation by Sponging MicroRNA-613. Cardiovasc. Ther. 2020, 2020, 5925342.
- Christophersen, I.E.; Holmegard, H.N.; Jabbari, J.; Haunsø, S.; Tveit, A.; Svendsen, J.H.; Olesen, M.S. Rare Variants in GJA5 Are Associated With Early-Onset Lone Atrial Fibrillation. Can. J. Cardiol. 2013, 29, 111–116.
- Santa Cruz, A.; Meşe, G.; Valiuniene, L.; Brink, P.R.; White, T.W.; Valiunas, V. Altered conductance and permeability of Cx40 mutations associated with atrial fibrillation. J. Gen. Physiol. 2015, 146, 387–398.
- Wirka, R.C.; Gore, S.; Van Wagoner, D.R.; Arking, D.E.; Lubitz, S.A.; Lunetta, K.L.; Benjamin, E.J.; Alonso, A.; Ellinor, P.T.; Barnard, J.; et al. A Common Connexin-40 Gene Promoter Variant Affects Connexin-40 Expression in Human Atria and Is Associated with Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2011, 4, 87–93.
- Nao, T.; Ohkusa, T.; Hisamatsu, Y.; Inoue, N.; Matsumoto, T.; Yamada, J.; Shimizu, A.; Yoshiga, Y.; Yamagata, T.; Kobayashi, S.; et al. Comparison of expression of connexin in right atrial myocardium in patients with chronic atrial fibrillation versus those in sinus rhythm. Am. J. Cardiol. 2003, 91, 678–683.
- Ghazizadeh, Z.; Kiviniemi, T.; Olafsson, S.; Plotnick, D.; Beerens, M.E.; Zhang, K.; Gillon, L.; Steinbaugh, M.J.; Barrera, V.; Sui, S.H.; et al. Metastable Atrial State Underlies the Primary Genetic Substrate for MYL4 Mutation-Associated Atrial Fibrillation. Circulation 2020, 141, 301–312.
- Dhein, S.; Rothe, S.; Busch, A.; Rojas Gomez, D.; Boldt, A.; Reutemann, A.; Seidel, T.; Salameh, A.; Pfannmüller, B.; Rastan, A.; et al. Effects of metoprolol therapy on cardiac gap junction remodelling and conduction in human chronic atrial fibrillation. Br. J. Pharmacol. 2011, 164, 607–616.
- Callegari, S.; Macchi, E.; Monaco, R.; Magnani, L.; Tafuni, A.; Croci, S.; Nicastro, M.; Garrapa, V.; Banchini, A.; Becchi, G.; et al. Clinicopathological Bird’s-Eye View of Left Atrial Myocardial Fibrosis in 121 Patients with Persistent Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2020, 13, e007588.
- Morgan, R.; Colman, M.A.; Chubb, H.; Seemann, G.; Aslanidi, O.V. Slow Conduction in the Border Zones of Patchy Fibrosis Stabilizes the Drivers for Atrial Fibrillation: Insights from Multi-Scale Human Atrial Modeling. Front. Physiol. 2016, 7, 474.
- Nakamura, T.; Kiuchi, K.; Fukuzawa, K.; Takami, M.; Watanabe, Y.; Izawa, Y.; Suehiro, H.; Akita, T.; Takemoto, M.; Sakai, J.; et al. Late-gadolinium enhancement properties associated with atrial fibrillation rotors in patients with persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2021, 32, 1005–1013.
- Zou, R.; Yang, M.; Shi, W.; Zheng, C.; Zeng, H.; Lin, X.; Zhang, D.; Yang, S.; Hua, P. Analysis of Genes Involved in Persistent Atrial Fibrillation: Comparisons of ‘Trigger’ and ‘Substrate’ Differences. Cell. Physiol. Biochem. 2018, 47, 1299–1309.
- Xiao, H.; Lei, H.; Qin, S.; Ma, K.; Wang, X. TGF-β1 Expression and Atrial Myocardium Fibrosis Increase in Atrial Fibrillation Secondary to Rheumatic Heart Disease. Clin. Cardiol. 2010, 33, 149–156.
- Su, L.; Yao, Y.; Song, W. Downregulation of miR-96 suppresses the profibrogenic functions of cardiac fibroblasts induced by angiotensin II and attenuates atrial fibrosis by upregulating KLF13. Hum. Cell 2020, 33, 337–346.
- Yu, R.-B.; Li, K.; Wang, G.; Gao, G.-M.; Du, J.-X. MiR-23 enhances cardiac fibroblast proliferation and suppresses fibroblast apoptosis via targeting TGF-β1 in atrial fibrillation. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4419–4424.
- Xu, J.; Lei, S.; Sun, S.; Zhang, W.; Zhu, F.; Yang, H.; Xu, Q.; Zhang, B.; Li, H.; Zhu, M.; et al. MiR-324-3p Regulates Fibroblast Proliferation via Targeting TGF-β1 in Atrial Fibrillation. Int. Heart J. 2020, 61, 1270–1278.
- Lu, J.; Xu, F.-Q.; Guo, J.-J.; Lin, P.-L.; Meng, Z.; Hu, L.-G.; Li, J.; Li, D.; Lu, X.-H.; An, Y. Long noncoding RNA GAS5 attenuates cardiac fibroblast proliferation in atrial fibrillation via repressing ALK5. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7605–7610.
- Wang, H.; Song, T.; Zhao, Y.; Zhao, J.; Wang, X.; Fu, X. Long non-coding RNA LICPAR regulates atrial fibrosis via TGF-β/Smad pathway in atrial fibrillation. Tissue Cell 2020, 67, 101440.
- Liu, Y.; Yin, Z.; Xu, X.; Liu, C.; Duan, X.; Song, Q.; Tuo, Y.; Wang, C.; Yang, J.; Yin, S. Crosstalk between the activated Slit2–Robo1 pathway and TGF-β1 signalling promotes cardiac fibrosis. ESC Heart Fail. 2021, 8, 447–460.
- Liu, Y.; Lv, H.; Tan, R.; An, X.; Niu, X.-H.; Liu, Y.-J.; Yang, X.; Yin, X.; Xia, Y.-L. Platelets Promote Ang II (Angiotensin II)-Induced Atrial Fibrillation by Releasing TGF-β1 (Transforming Growth Factor-β1) and Interacting with Fibroblasts. Hypertension 2020, 76, 1856–1867.
- Jiang, Z.; Zhong, G.; Wen, L.; Hong, Y.; Fang, S.; Sun, P.; Li, S.; Li, S.; Feng, G. The Role of Platelet-Derived Growth Factor-B/Platelet-Derived Growth Factor Receptor-β Signaling in Chronic Atrial Fibrillation. Cardiology 2016, 133, 242–256.
- Yang, D.; Yuan, J.; Liu, G.; Ling, Z.; Zeng, H.; Chen, Y.; Zhang, Y.; She, Q.; Zhou, X. Angiotensin Receptor Blockers and Statins Could Alleviate Atrial Fibrosis via Regulating Platelet-Derived Growth Factor/Rac1 /Nuclear Factor-Kappa B Axis. Int. J. Med. Sci. 2013, 10, 812–824.
- Liu, L.; Luo, F.; Lei, K. Exosomes Containing LINC00636 Inhibit MAPK1 through the miR-450a-2-3p Overexpression in Human Pericardial Fluid and Improve Cardiac Fibrosis in Patients with Atrial Fibrillation. Mediat. Inflamm. 2021, 2021, 9960241.
- Zheng, L.; Jia, X.; Zhang, C.; Wang, D.; Cao, Z.; Wang, J.; Du, X. Angiotensin II in atrial structural remodeling: The role of Ang II/JAK/STAT3 signaling pathway. Am. J. Transl. Res. 2015, 7, 1021–1031.
- Boldt, A.; Wetzel, U.; Weigl, J.; Garbade, J.; Lauschke, J.; Hindricks, G.; Kottkamp, H.; Gummert, J.F.; Dhein, S. Expression of angiotensin II receptors in human left and right atrial tissue in atrial fibrillation with and without underlying mitral valve disease. J. Am. Coll. Cardiol. 2003, 42, 1785–1792.
- Gassanov, N.; Brandt, M.C.; Michels, G.; Lindner, M.; Er, F.; Hoppe, U.C. Angiotensin II-induced changes of calcium sparks and ionic currents in human atrial myocytes: Potential role for early remodeling in atrial fibrillation. Cell Calcium 2006, 39, 175–186.
- Dong, Q.; Li, S.; Wang, W.; Han, L.; Xia, Z.; Wu, Y.; Tang, Y.; Li, J.; Cheng, X. FGF23 regulates atrial fibrosis in atrial fibrillation by mediating the STAT3 and SMAD3 pathways. J. Cell. Physiol. 2019, 234, 19502–19510.
- Chen, J.; Guo, Y.; Chen, Q.; Cheng, X.; Xiang, G.; Chen, M.; Wu, H.; Huang, Q.; Zhu, P.; Zhang, J. TGFβ1 and HGF regulate CTGF expression in human atrial fibroblasts and are involved in atrial remodelling in patients with rheumatic heart disease. J. Cell. Mol. Med. 2019, 23, 3032–3039.
- Wang, Q.; Xi, W.; Yin, L.; Wang, J.; Shen, H.; Gao, Y.; Min, J.; Zhang, Y.; Wang, Z. Human Epicardial Adipose Tissue cTGF Expression is an Independent Risk Factor for Atrial Fibrillation and Highly Associated with Atrial Fibrosis. Sci. Rep. 2018, 8, 3585.
- Qiao, G.; Xia, D.; Cheng, Z.; Zhang, G. miR-132 in atrial fibrillation directly targets connective tissue growth factor. Mol. Med. Rep. 2017, 16, 4143–4150.
- Ko, W.-C.; Hong, C.-Y.; Hou, S.-M.; Lin, C.-H.; Ong, E.-T.; Lee, C.-F.; Tsai, C.-T.; Lai, L.-P. Elevated Expression of Connective Tissue Growth Factor in Human Atrial Fibrillation and Angiotensin II-Treated Cardiomyocytes. Circ. J. 2011, 75, 1592–1600.
- Moreira, L.M.; Takawale, A.; Hulsurkar, M.; Menassa, D.A.; Antanaviciute, A.; Lahiri, S.K.; Mehta, N.; Evans, N.; Psarros, C.; Robinson, P.; et al. Paracrine signalling by cardiac calcitonin controls atrial fibrogenesis and arrhythmia. Nature 2020, 587, 460–465.
- Jakob, D.; Klesen, A.; Darkow, E.; Kari, F.A.; Beyersdorf, F.; Kohl, P.; Ravens, U.; Peyronnet, R. Heterogeneity and Remodeling of Ion Currents in Cultured Right Atrial Fibroblasts From Patients With Sinus Rhythm or Atrial Fibrillation. Front. Physiol. 2021, 12.
- Ashihara, T.; Haraguchi, R.; Nakazawa, K.; Namba, T.; Ikeda, T.; Nakazawa, Y.; Ozawa, T.; Ito, M.; Horie, M.; Trayanova, N.A. The Role of Fibroblasts in Complex Fractionated Electrograms During Persistent/Permanent Atrial Fibrillation. Circ. Res. 2012, 110, 275–284.
- Sánchez, J.; Gomez, J.F.; Martinez-Mateu, L.; Romero, L.; Saiz, J.; Trenor, B. Heterogeneous Effects of Fibroblast-Myocyte Coupling in Different Regions of the Human Atria Under Conditions of Atrial Fibrillation. Front. Physiol. 2019, 10, 847.
- Nagashima, K.; Okumura, Y.; Watanabe, I.; Nakai, T.; Ohkubo, K.; Kofune, M.; Mano, H.; Sonoda, K.; Hiro, T.; Nikaido, M.; et al. Does Location of Epicardial Adipose Tissue Correspond to Endocardial High Dominant Frequency or Complex Fractionated Atrial Electrogram Sites During Atrial Fibrillation? Circ. Arrhythmia Electrophysiol. 2012, 5, 676–683.
- Zghaib, T.; Ipek, E.G.; Zahid, S.; Balouch, M.A.; Misra, S.; Ashikaga, H.; Berger, R.D.; Marine, J.E.; Spragg, D.D.; Zimmerman, S.L.; et al. Association of left atrial epicardial adipose tissue with electrogram bipolar voltage and fractionation: Electrophysiologic substrates for atrial fibrillation. Heart Rhythm 2016, 13, 2333–2339.
- Mahajan, R.; Nelson, A.; Pathak, R.K.; Middeldorp, M.E.; Wong, C.X.; Twomey, D.J.; Carbone, A.; Teo, K.; Agbaedeng, T.; Linz, D.; et al. Electroanatomical Remodeling of the Atria in Obesity. JACC Clin. Electrophysiol. 2018, 4, 1529–1540.
- Nalliah, C.J.; Bell, J.R.; Raaijmakers, A.J.A.; Waddell, H.M.; Wells, S.P.; Bernasochi, G.B.; Montgomery, M.K.; Binny, S.; Watts, T.; Joshi, S.B.; et al. Epicardial Adipose Tissue Accumulation Confers Atrial Conduction Abnormality. J. Am. Coll. Cardiol. 2020, 76, 1197–1211.
- Shaihov-Teper, O.; Ram, E.; Ballan, N.; Brzezinski, R.Y.; Naftali-Shani, N.; Masoud, R.; Ziv, T.; Lewis, N.; Schary, Y.; Levin-Kotler, L.-P.; et al. Extracellular Vesicles From Epicardial Fat Facilitate Atrial Fibrillation. Circulation 2021, 143, 2475–2493.
- Suffee, N.; Moore-Morris, T.; Farahmand, P.; Rücker-Martin, C.; Dilanian, G.; Fradet, M.; Sawaki, D.; Derumeaux, G.; LePrince, P.; Clément, K.; et al. Atrial natriuretic peptide regulates adipose tissue accumulation in adult atria. Proc. Natl. Acad. Sci. USA 2017, 114, E771–E780.
- Liu, Q.; Zhang, F.; Yang, M.; Zhong, J. Increasing Level of Interleukin-1β in Epicardial Adipose Tissue Is Associated with Persistent Atrial Fibrillation. J. Interf. Cytokine Res. 2020, 40, 64–69.
- Couselo-Seijas, M.; Lopez-Canoa, J.N.; Fernandez, Á.L.; González-Melchor, L.; Seoane, L.M.; Duran-Muñoz, D.; Rozados-Luis, A.; González-Juanatey, J.R.; Rodríguez-Mañero, M.; Eiras, S. Inflammatory and lipid regulation by cholinergic activity in epicardial stromal cells from patients who underwent open-heart surgery. J. Cell. Mol. Med. 2020, 24, 10958–10969.
- Kusayama, T.; Furusho, H.; Kashiwagi, H.; Kato, T.; Murai, H.; Usui, S.; Kaneko, S.; Takamura, M. Inflammation of left atrial epicardial adipose tissue is associated with paroxysmal atrial fibrillation. J. Cardiol. 2016, 68, 406–411.
- Haemers, P.; Hamdi, H.; Guedj, K.; Suffee, N.; Farahmand, P.; Popovic, N.; Claus, P.; LePrince, P.; Nicoletti, A.; Jalife, J.; et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. 2017, 38, 53–61.
- Kira, S.; Abe, I.; Ishii, Y.; Miyoshi, M.; Oniki, T.; Arakane, M.; Daa, T.; Teshima, Y.; Yufu, K.; Shimada, T.; et al. Role of angiopoietin-like protein 2 in atrial fibrosis induced by human epicardial adipose tissue: Analysis using an organo-culture system. Heart Rhythm 2020, 17, 1591–1601.
- Chen, Y.; Liu, F.; Han, F.; Lv, L.; Tang, C.; Xie, Z.; Luo, F. Omentin-1 is associated with atrial fibrillation in patients with cardiac valve disease. BMC Cardiovasc. Disord. 2020, 20, 214.
- Venteclef, N.; Guglielmi, V.; Balse, E.; Gaborit, B.; Cotillard, A.; Atassi, F.; Amour, J.; Leprince, P.; Dutour, A.; Clément, K.; et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur. Heart J. 2015, 36, 795–805.
- Wang, Q.; Shen, H.; Min, J.; Gao, Y.; Liu, K.; Xi, W.; Yang, J.; Yin, L.; Xu, J.; Xiao, J.; et al. YKL-40 is highly expressed in the epicardial adipose tissue of patients with atrial fibrillation and associated with atrial fibrosis. J. Transl. Med. 2018, 16, 229.
- Aksu, T.; Yalin, K.; Bozyel, S.; Gopinathannair, R.; Gupta, D. The anatomical basis behind the neuromodulation effects associated with pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2021, 32, 1733–1736.
- Pachon, J.C.; Pachon, E.I.; Pachon, C.T.C.; Santillana, T.G.; Lobo, T.J.; Pachon, J.C.; Zerpa, J.C.; Cunha, M.Z.; Higuti, C.; Ortencio, F.A.; et al. Long-Term Evaluation of the Vagal Denervation by Cardioneuroablation Using Holter and Heart Rate Variability. Circ. Arrhythmia Electrophysiol. 2020, 13, e008703.
- Zhu, Z.; Wang, W.; Cheng, Y.; Wang, X.; Sun, J. The predictive value of heart rate variability indices tested in early period after radiofrequency catheter ablation for the recurrence of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2020, 31, 1350–1355.
- Kusayama, T.; Douglas, A.; Wan, J.; Doytchinova, A.; Wong, J.; Mitscher, G.; Straka, S.; Shen, C.; Everett, T.H.; Chen, P.-S. Skin sympathetic nerve activity and ventricular rate control during atrial fibrillation. Heart Rhythm 2020, 17, 544–552.
- Kawasaki, M.; Yamada, T.; Furukawa, Y.; Morita, T.; Tamaki, S.; Kida, H.; Sakata, Y.; Fukunami, M. Are cardiac sympathetic nerve activity and epicardial adipose tissue associated with atrial fibrillation recurrence after catheter ablation in patients without heart failure? Int. J. Cardiol. 2020, 303, 41–48.
- Kampaktsis, P.N.; Oikonomou, E.K.; Choi, D.Y.; Cheung, J.W. Efficacy of ganglionated plexi ablation in addition to pulmonary vein isolation for paroxysmal versus persistent atrial fibrillation: A meta-analysis of randomized controlled clinical trials. J. Interv. Card. Electrophysiol. 2017, 50, 253–260.
- Waldron, N.H.; Cooter, M.; Haney, J.C.; Schroder, J.N.; Gaca, J.G.; Lin, S.S.; Sigurdsson, M.I.; Fudim, M.; Podgoreanu, M.V.; Stafford-Smith, M.; et al. Temporary autonomic modulation with botulinum toxin type A to reduce atrial fibrillation after cardiac surgery. Heart Rhythm 2019, 16, 178–184.
- Romanov, A.; Pokushalov, E.; Ponomarev, D.; Bayramova, S.; Shabanov, V.; Losik, D.; Stenin, I.; Elesin, D.; Mikheenko, I.; Strelnikov, A.; et al. Long-term suppression of atrial fibrillation by botulinum toxin injection into epicardial fat pads in patients undergoing cardiac surgery: Three-year follow-up of a randomized study. Heart Rhythm 2019, 16, 172–177.
- Wang, H.; Zhang, Y.; Xin, F.; Jiang, H.; Tao, D.; Jin, Y.; He, Y.; Wang, Q.; Po, S.S. Calcium-Induced Autonomic Denervation in Patients with Post-Operative Atrial Fibrillation. J. Am. Coll. Cardiol. 2021, 77, 57–67.
- Stavrakis, S.; Stoner, J.A.; Humphrey, M.B.; Morris, L.; Filiberti, A.; Reynolds, J.C.; Elkholey, K.; Javed, I.; Twidale, N.; Riha, P.; et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation). JACC Clin. Electrophysiol. 2020, 6, 282–291.
- Wu, J.; Deng, H.; Chen, Q.; Wu, Q.; Li, X.; Jiang, S.; Wang, F.; Ye, F.; Ou, L.; Gao, H. Comprehensive Analysis of Differential Immunocyte Infiltration and Potential ceRNA Networks Involved in the Development of Atrial Fibrillation. BioMed Res. Int. 2020, 2020, 8021208.
- Hohmann, C.; Pfister, R.; Mollenhauer, M.; Adler, C.; Kozlowski, J.; Wodarz, A.; Drebber, U.; Wippermann, J.; Michels, G. Inflammatory cell infiltration in left atrial appendageal tissues of patients with atrial fibrillation and sinus rhythm. Sci. Rep. 2020, 10, 1685.
- Yamashita, T.; Sekiguchi, A.; Iwasaki, Y.; Date, T.; Sagara, K.; Tanabe, H.; Suma, H.; Sawada, H.; Aizawa, T. Recruitment of Immune Cells Across Atrial Endocardium in Human Atrial Fibrillation. Circ. J. 2010, 74, 262–270.
- Smorodinova, N.; Bláha, M.; Melenovský, V.; Rozsívalová, K.; Přidal, J.; Ďurišová, M.; Pirk, J.; Kautzner, J.; Kučera, T. Analysis of immune cell populations in atrial myocardium of patients with atrial fibrillation or sinus rhythm. PLoS ONE 2017, 12, e0172691.
- Chen, Y.; Chang, G.; Chen, X.; Li, Y.; Li, H.; Cheng, D.; Tang, Y.; Sang, H. IL-6-miR-210 Suppresses Regulatory T Cell Function and Promotes Atrial Fibrosis by Targeting Foxp3. Mol. Cells 2020, 43, 438–447.
- Rao, F.; Deng, C.-Y.; Wu, S.-L.; Xiao, D.-Z.; Yu, X.-Y.; Kuang, S.-J.; Lin, Q.-X.; Shan, Z.-X. Involvement of Src in L-type Ca2+ channel depression induced by macrophage migration inhibitory factor in atrial myocytes. J. Mol. Cell. Cardiol. 2009, 47, 586–594.
- Ambale-Venkatesh, B.; Yang, X.; Wu, C.O.; Liu, K.; Hundley, W.G.; McClelland, R.; Gomes, A.S.; Folsom, A.R.; Shea, S.; Guallar, E.; et al. Cardiovascular Event Prediction by Machine Learning. Circ. Res. 2017, 121, 1092–1101.
- Pan, J.; Wang, W.; Wu, X.; Kong, F.; Pan, J.; Lin, J.; Zhang, M. Inflammatory cytokines in cardiac pacing patients with atrial fibrillation and asymptomatic atrial fibrillation. Panminerva Med. 2018, 60, 86–91.
- Maida, C.D.; Vasto, S.; Di Raimondo, D.; Casuccio, A.; Vassallo, V.; Daidone, M.; Del Cuore, A.; Pacinella, G.; Cirrincione, A.; Simonetta, I.; et al. Inflammatory activation and endothelial dysfunction markers in patients with permanent atrial fibrillation: A cross-sectional study. Aging 2020, 12, 8423–8433.
- De Gennaro, L.; Brunetti, N.D.; Montrone, D.; De Rosa, F.; Cuculo, A.; Di Biase, M. Inflammatory activation and carbohydrate antigen-125 levels in subjects with atrial fibrillation. Eur. J. Clin. Investig. 2012, 42, 371–375.
- Liuba, I.; Ahlmroth, H.; Jonasson, L.; Englund, A.; Jonsson, A.; Safstrom, K.; Walfridsson, H. Source of inflammatory markers in patients with atrial fibrillation. Europace 2008, 10, 848–853.
- Lin, Y.-J.; Tsao, H.-M.; Chang, S.-L.; Lo, L.-W.; Tuan, T.-C.; Hu, Y.-F.; Udyavar, A.R.; Tsai, W.-C.; Chang, C.-J.; Tai, C.-T.; et al. Prognostic Implications of the High-Sensitive C-Reactive Protein in the Catheter Ablation of Atrial Fibrillation. Am. J. Cardiol. 2010, 105, 495–501.
- Yao, C.; Veleva, T.; Scott, L.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242.
- Heijman, J.; Muna, A.P.; Veleva, T.; Molina, C.E.; Sutanto, H.; Tekook, M.; Wang, Q.; Abu-Taha, I.H.; Gorka, M.; Künzel, S.; et al. Atrial myocyte NLRP3/CaMKII nexus forms a substrate for postoperative atrial fibrillation. Circ. Res. 2020, 127, 1036–1055.
- Liu, C.; Wang, J.; Yiu, D.; Liu, K. The Efficacy of Glucocorticoids for the Prevention of Atrial Fibrillation, or Length of Intensive Care Unite or Hospital Stay After Cardiac Surgery: A Meta-Analysis. Cardiovasc. Ther. 2014, 32, 89–96.
- Liu, L.; Jing, F.-Y.; Wang, X.-W.; Li, L.-J.; Zhou, R.-Q.; Zhang, C.; Wu, Q.-C. Effects of corticosteroids on new-onset atrial fibrillation after cardiac surgery. Medicine 2021, 100, e25130.
- Papageorgiou, N.; Briasoulis, A.; Lazaros, G.; Imazio, M.; Tousoulis, D. Colchicine for prevention and treatment of cardiac diseases: A meta-analysis. Cardiovasc. Ther. 2017, 35, 10–18.
- Deftereos, S.G.; Vrachatis, D.A.; Angelidis, C.; Vrettou, A.-R.; Sarri, E.K.; Giotaki, S.G.; Varytimiadi, E.; Kossyvakis, C.; Kotsia, E.; Deftereos, G.S.; et al. The Role of Colchicine in Treating Postoperative and Post-catheter Ablation Atrial Fibrillation. Clin. Ther. 2019, 41, 21–29.
- Sabath, N.; Levy-Adam, F.; Younis, A.; Rozales, K.; Meller, A.; Hadar, S.; Soueid-Baumgarten, S.; Shalgi, R. Cellular proteostasis decline in human senescence. Proc. Natl. Acad. Sci. USA 2020, 117, 31902–31913.
- Arrieta, A.; Blackwood, E.A.; Stauffer, W.T.; Glembotski, C.C. Integrating ER and Mitochondrial Proteostasis in the Healthy and Diseased Heart. Front. Cardiovasc. Med. 2020, 6, 193.
- Brundel, B.J.J.M.; Henning, R.H.; Ke, L.; van Gelder, I.C.; Crijns, H.J.G.M.; Kampinga, H.H. Heat shock protein upregulation protects against pacing-induced myolysis in HL-1 atrial myocytes and in human atrial fibrillation. J. Mol. Cell. Cardiol. 2006, 41, 555–562.
- Hu, Y.-F.; Yeh, H.-I.; Tsao, H.-M.; Tai, C.-T.; Lin, Y.-J.; Chang, S.-L.; Lo, L.-W.; Tuan, T.-C.; Suenari, K.; Li, C.-H.; et al. Electrophysiological Correlation and Prognostic Impact of Heat Shock Protein 27 in Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2012, 5, 334–340.
- Marion, D.; Lanters, E.A.; Ramos, K.S.; Li, J.; Wiersma, M.; Baks-te Bulte, L.; JQMMuskens, A.; Boersma, E.; de Groot, N.; Brundel, B.J. Evaluating Serum Heat Shock Protein Levels as Novel Biomarkers for Atrial Fibrillation. Cells 2020, 9, 2105.
- Wiersma, M.; Meijering, R.A.M.; Qi, X.; Zhang, D.; Liu, T.; Hoogstra-Berends, F.; Sibon, O.C.M.; Henning, R.H.; Nattel, S.; Brundel, B.J.J.M. Endoplasmic Reticulum Stress Is Associated with Autophagy and Cardiomyocyte Remodeling in Experimental and Human Atrial Fibrillation. J. Am. Heart Assoc. 2017, 6, e006458.
- Brundel, B. Activation of proteolysis by calpains and structural changes in human paroxysmal and persistent atrial fibrillation. Cardiovasc. Res. 2002, 54, 380–389.
- Zhang, D.; Wu, C.-T.; Qi, X.; Meijering, R.A.M.; Hoogstra-Berends, F.; Tadevosyan, A.; Cubukcuoglu Deniz, G.; Durdu, S.; Akar, A.R.; Sibon, O.C.M.; et al. Activation of Histone Deacetylase-6 Induces Contractile Dysfunction Through Derailment of α-Tubulin Proteostasis in Experimental and Human Atrial Fibrillation. Circulation 2014, 129, 346–358.
- Sawa, Y.; Matsushita, N.; Sato, S.; Ishida, N.; Saito, M.; Sanbe, A.; Morino, Y.; Taira, E.; Obara, M.; Hirose, M. Chronic HDAC6 Activation Induces Atrial Fibrillation Through Atrial Electrical and Structural Remodeling in Transgenic Mice. Int. Heart J. 2021, 62, 616–626.
- Zhang, D.; Hu, X.; Li, J.; Liu, J.; Baks-te Bulte, L.; Wiersma, M.; Malik, N.-A.; van Marion, D.M.S.; Tolouee, M.; Hoogstra-Berends, F.; et al. DNA damage-induced PARP1 activation confers cardiomyocyte dysfunction through NAD+ depletion in experimental atrial fibrillation. Nat. Commun. 2019, 10, 1307.
- Kim, Y.M.; Guzik, T.J.; Zhang, Y.H.; Zhang, M.H.; Kattach, H.; Ratnatunga, C.; Pillai, R.; Channon, K.M.; Casadei, B. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ. Res. 2005, 97, 629–636.
- Liu, T.; Shao, Q.; Korantzopoulos, P.; Liu, E.; Xu, G.; Li, G. Serum levels of nicotinamide-adenine dinucleotide phosphate oxidase 4 are associated with non-valvular atrial fibrillation. Biomed. Rep. 2015, 3, 864–868.
- Li, J.; Zhang, D.; Ramos, K.S.; Baks, L.; Wiersma, M.; Lanters, E.A.H.; Bogers, A.J.J.C.; de Groot, N.M.S.; Brundel, B.J.J.M. Blood-based 8-hydroxy-2′-deoxyguanosine level: A potential diagnostic biomarker for atrial fibrillation. Heart Rhythm 2021, 18, 271–277.
- Toyama, K.; Yamabe, H.; Uemura, T.; Nagayoshi, Y.; Morihisa, K.; Koyama, J.; Kanazawa, H.; Hoshiyama, T.; Ogawa, H. Analysis of oxidative stress expressed by urinary level of 8-hydroxy-2′-deoxyguanosine and biopyrrin in atrial fibrillation: Effect of sinus rhythm restoration. Int. J. Cardiol. 2013, 168, 80–85.
- Wiersma, M.; van Marion, D.; Wüst, R.C.; Houtkooper, R.H.; Zhang, D.; de Groot, N.; Henning, R.H.; Brundel, B.J. Mitochondrial Dysfunction Underlies Cardiomyocyte Remodeling in Experimental and Clinical Atrial Fibrillation. Cells 2019, 8, 1202.
- Emelyanova, L.; Ashary, Z.; Cosic, M.; Negmadjanov, U.; Ross, G.; Rizvi, F.; Olet, S.; Kress, D.; Sra, J.; Tajik, A.J.; et al. Selective downregulation of mitochondrial electron transport chain activity and increased oxidative stress in human atrial fibrillation. Am. J. Physiol. Circ. Physiol. 2016, 311, H54–H63.
- Montaigne, D.; Marechal, X.; Lefebvre, P.; Modine, T.; Fayad, G.; Dehondt, H.; Hurt, C.; Coisne, A.; Koussa, M.; Remy-Jouet, I.; et al. Mitochondrial Dysfunction as an Arrhythmogenic Substrate. J. Am. Coll. Cardiol. 2013, 62, 1466–1473.
- Chen, G.; Guo, H.; Song, Y.; Chang, H.; Wang, S.; Zhang, M.; Liu, C. Long non-coding RNA AK055347 is upregulated in patients with atrial fibrillation and regulates mitochondrial energy production in myocardiocytes. Mol. Med. Rep. 2016, 14, 5311–5317.
- Xie, W.; Santulli, G.; Reiken, S.R.; Yuan, Q.; Osborne, B.W.; Chen, B.-X.; Marks, A.R. Mitochondrial oxidative stress promotes atrial fibrillation. Sci. Rep. 2015, 5, 11427.
- Harada, M.; Tadevosyan, A.; Qi, X.; Xiao, J.; Liu, T.; Voigt, N.; Karck, M.; Kamler, M.; Kodama, I.; Murohara, T.; et al. Atrial Fibrillation Activates AMP-Dependent Protein Kinase and its Regulation of Cellular Calcium Handling. J. Am. Coll. Cardiol. 2015, 66, 47–58.
