The clinical spectrum of prostate cancer (PCa) varies from castration-naive to metastatic castration-resistant disease. Despite the administration of androgen synthesis inhibitors and chemotherapy regimens for castration-resistant prostate cancer, the treatment options for this entity are limited. The utilization of the immune system against cancer cells shows potential as a therapeutic modality for various solid tumors and hematologic malignancies. With technological advances over the last decade, immunotherapy has become an integral treatment modality for advanced solid tumors. The feasibility of immunotherapy has shown promise for patients with PCa, and with advances in molecular diagnostic platforms and our understanding of immune mechanisms, immunotherapy is reemerging as a potential treatment modality for PCa. Various combinations of individualized immunotherapy and immune checkpoint blockers with androgen receptor-targeted therapies and conventional cytotoxic agents show promise. This article will review the current status of immunotherapy, including new discoveries and precision approaches for PCa, and discuss future directions in the continuously evolving landscape of immunotherapy.
- prostate cancer
- checkpoint inhibitor
- immunology
- Introduction
Prostate cancer (PCa) is the most commonly diagnosed malignancy among men and the second most common cause of cancer-associated death in industrialized nations [1]. The standard primary treatments for localized PCa are radical prostatectomy or radiation therapy (RT) with or without androgen-deprivation therapy (ADT), while the primary treatment modality for PCa patients in the advanced setting is ADT. Despite initial therapeutic responses with ADT, the majority of patients are destined to progress to metastatic castration-resistant PCa (mCRPC) [2]. Therefore, novel therapeutic approaches with durable response rates for advanced disease are warranted.
Current agents approved by the US Food and Drug Administration (FDA) that have shown efficacy in terms of overall survival (OS) in patients with mCRPC include docetaxel, cabazitaxel, radium-223, sipuleucel-T, and androgen receptor axis-targeted agents including abiraterone and enzalutamide [3–8]. Since the approval of sipuleucel-T in 2010 [4], there have been significant advancements and innovations in the field of immunotherapy. However, with the exception of sipuleucel-T, no other immunotherapeutic treatment has been approved for the treatment of mCRPC, owing to limited response rates and modest clinical efficacy. Nevertheless, with the introduction of next-generation genomic diagnostic platforms and advancements in our understanding of the molecular pathophysiology, we are now observing a considerable shift in the paradigm of immunotherapy for the treatment of PCa.
This review will highlight the re-emergence of immunotherapeutic approaches in the treatment of PCa, mainly focusing on immune checkpoint inhibitors (ICIs), which have shown the potential to revolutionize the treatment of both localized and advanced PCa.
- Pathophysiology of Prostate Cancer
The pathogenesis of PCa is a slow, ongoing progression that involves small developing cancers that gradually evolve into different clonal entities with various clinical outcomes [9–11]. Previous studies have shown that chronic inflammation is frequent in the prostates of elderly men and that this change is associated with an increased risk of PCa development [12,13]. The underlying mechanisms of chronic prostate inflammation and its clinical relevance to the development of PCa are unclear. Nevertheless, clinical evidence shows that chronic inflammation is a potential risk factor for disease progression and poor clinical outcomes [14–17].
Cellular pathways that modulate the proliferation, migration, and survival of cancer cells are upregulated through cytokines and signaling molecules, including tumor necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β), C-C motif chemokine ligand 2 (CCL-2), and interleukins (IL-2, IL-6, IL-8, and IL-10) [17]. T cell activity and the associated autoimmune reaction induce epithelial and stromal cell proliferation. Infiltrating B cells are associated with increased antibody production, and recent studies have reported that specific subsets, namely regulatory B cells, may also play a significant role in tumor progression [18–20]. Moreover, increased B cell infiltrates have been observed in PCa [21]. Although T and B cells are the immune cell types that are predominantly observed in PCa, various ILs and inflammatory cell cytokines that dwell in the tumor stroma, such as granulocytes, macrophages, natural killer (NK) cells, myeloid-derived suppressor cells (MDSCs), and monocytes, additionally play an essential role in promoting the autocrine or paracrine proliferation of PCa cells [22–25].
Along with the aforementioned immune cells, androgen receptors (ARs) are a crucial factor in the tumorigenesis of PCa. The AR is a ligand-dependent transcription factor that is densely populated in thymic epithelial cells (TECs) and is essential for the development and function of male accessory sex organs [26,27]. ARs have a crucial role in the proliferation of PCa cells. The downregulation of gene expression required for regular TEC activity results in decreased thymocyte proliferation and an increase in apoptosis, which ultimately leads to thymic involution [27]. AR signaling directly affects the activity of circulating T cells by increasing the transcription of the protein tyrosine phosphatase non-receptor type 1 (PTPN 1). This downregulates the Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway and subsequently suppress T helper type 1 (Th1) cell differentiation [28]. Moreover, androgen cessation can inversely affect the involution of the thymus by upregulating thymocyte proliferation and differentiation and can subsequently stimulate T cell infiltration [29,30]. Evidence from numerous studies has shown that PCa cells consist of a highly reactive stroma, which is featured by upregulated T cell infiltrates densely populated with regulatory T cells that have immunosuppressive tendencies [31,32].
As integral members of the innate immune system, myeloid cells can be stratified into two individual groups. The first group consists of mononuclear cells such as dendritic cells, macrophages, and monocytes, and the second consists of polymorphonuclear cells, which include basophils, eosinophils, mast cells, and neutrophils. Many studies have shown a tumor-promoting function of terminally differentiated myeloid-derived cells, as they can promote cancer cell proliferation and migration, tumor angiogenesis, and immune suppression [33–37]. In the PCa microenvironment, the cessation of androgen induces CCL2 upregulation and activation of the STAT 3 pathway, which initiates the recruitment of macrophages and induces polarization of M2 macrophages [38–40]. Activated M1 macrophages have the ability to promote a Th1 response and can exert an efficient antigen-presenting function with the capability of terminating tumors. However, by initiating the proliferation of epithelial and stromal cells, neovascularization, and suppression of the immune system, the anti-inflammatory characteristics of M2 macrophages stimulate the Th2 response and tissue remodeling, all of which creates favorable changes in the microenvironment for disease progression [41].
- Immunotherapy Resistance of Prostate Cancer
Various clinical trials of PCa immunotherapy have been targeted at metastatic disease, with specific advances being made for novel therapies for CRPC. Patients with metastatic PCa have a dysfunctional and compromised immune system [42]. The immunogenicity of the cancer cells is the main determining factor in the immunotherapeutic approach to tumor eradication. Cancer cells are integrated into the self-immune system and, therefore, do not express foreign antigens. However, cancer cells exhibit tumor self-antigens, which can be immunogenic. Tumor neoantigens are the result of somatic mutations that are amassed in dividing cancer cells and are clinically correlated with the degree of mutation [43]. The load of tumor mutation has been associated with clinical outcomes of immunotherapy; however, tumor immunogenicity is influenced by numerous components that are regulated by the cancer cells [44–49].
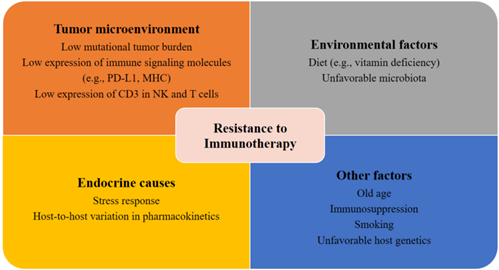
Patients with metastatic PCa are known to have disrupted cellular immunity as well as a tumor microenvironment with increased immunosuppressive qualities. The compromised immune system of patients with PCa is characterized by a reduction in NK cell activity and renewal and inhibited expression of CD3 in NK and T cells, which may ultimately result in the reduction of T cell receptors and NK cell-activating receptors [50–52]. Moreover, patients with mCRPC also have a reduced number of total T cells [53] and an increased number of myeloid suppressor cells and regulatory T cells in the tumor microenvironment and circulation [54–56]. Another potential explanation for the resistance and tolerance to immunotherapy in PCa is the slow disease progression [57,58]. The low mutational tumor burden in patients with PCa may contribute to de novo immunotherapy resistance (Figure 1) [59]. This view, however, is still under debate, as a recent genomic analysis indicated that patients with PCa had a higher tumor mutation burden than patients with renal cell carcinoma [60].
Further research is warranted to elucidate the exact pathological mechanism underlying the resistance of patients with PCa to immunotherapy to pave the way for an effective immunotherapeutic regimen for metastatic PCa.
Figure 1. Factors contributing to resistance to immunotherapy. Several potential tumor-related, host-related, and environmental factors may elicit resistance to immunotherapies.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21155484