Cardiotoxicity is a well-recognized late effect among childhood cancer survivors. With various pediatric cancers becoming increasingly curable, it is imperative to understand the disease burdens that survivors may face in the future.
1. Introduction
Heart failure can be an early or late cardiotoxic side effect of cancer treatment in children. Anthracycline cardiotoxicity is characterized as acute if it occurs within the first week of treatment, early-onset progressive cardiotoxicity if it occurs within the first year of treatment completion, and late-onset progressive cardiotoxicity if it occurs more than one year after the completion of treatment [1].
2. Pathophysiology of Cardiotoxic Cancer Therapies
2.1. Anthracyclines
Among antineoplastic agents, anthracyclines are best-known for contributing to cardiotoxicity in childhood cancer survivors. This family of agents includes doxorubicin, daunorubicin, epirubicin, and idarubicin. In a landmark study in adults, Swain et al. reported an estimated cumulative percentage of doxorubicin-related congestive heart failure of 5% of patients who received a cumulative dose of 400 mg/m
2 of doxorubicin, 26% of patients who received 550 mg/m
2 of doxorubicin, and 48% of patients who received 700 mg/m
2 of doxorubicin [
10]. Reports from the Childhood Cancer Survivorship study have demonstrated a clear risk above cumulative doses of 250 mg/m
2 but suggest that there may be no safe dose [
5]. While there are many purported hypotheses of anthracyclines’ mechanisms of toxicity, a complete understanding of anthracycline-induced cardiomyopathy does not yet exist. Some of the more common implicated mechanisms will be briefly summarized here.
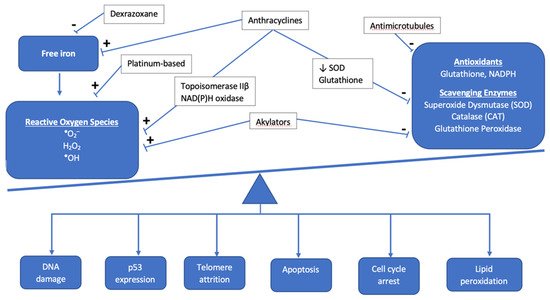
Anthracyclines increase cellular oxidative stress, which is associated with an imbalance between the creation of reactive oxygen species (ROS) and scavenging enzymes or antioxidants that normally keep ROS in check (
Figure 1). Elevated ROS leads to oxidative stress as electrons are scavenged to reach a more stable state, leading to DNA damage, lipid membrane peroxidation, cell cycle arrest, telomere attrition, and apoptosis [
11]. The most commonly used anthracycline in pediatric oncology is doxorubicin, which exerts its anti-neoplastic effect via topoisomerase IIα binding to DNA, inducing cell death. While tumor cells are rich in topoisomerase IIα, cardiomyocytes exhibit the topoisomerase IIβ isoform, which is thought to regulate genes involved in mitochondrial biogenesis and function [
12]. Under doxorubicin’s influence, oxidative phosphorylation within the mitochondria is impacted and leads to an imbalance in favor of increased ROS, ultimately leading to cardiotoxicity [
13]. Mitochondrial dysfunction also results from anthracyclines forming complexes with the inner mitochondrial membrane phospholipid cardiolipin, thereby disrupting electron transport chain activity and resulting in a shift to increased reliance on glucose metabolism in cardiomyocytes, which is a final common pathway in heart failure [
14,
15].
Figure 1. Overview of the interaction between antineoplastic chemotherapy and the role of oxidative stress in development of cardiotoxicity. The cumulative effect of chemotherapeutic agents results in the accumulation of reactive oxygen species and other mediators of oxidative stress, such as free iron, as antioxidants and scavenging enzymes are prevented from maintaining proper balance as they normally do under healthy physiologic conditions.
In addition to oxidative stress, there are other mechanisms of cellular apoptosis and cell injury mediated by anthracyclines. Anthracyclines cause direct DNA damage through the cleavage of DNA strands, but they also inhibit DNA biosynthesis and the enzymes involved in DNA repair [
16]. Anthracyclines increase the likelihood that calcium channels in the sarcoplasmic reticulum are in the open state, resulting in an increase in calcium release [
17,
18]. Sustained calcium leak leads to further ROS production and ultimately culminates in the activation of the caspase cascade and cellular apoptosis. Anthracyclines result in impaired iron sequestration and, therefore, an increase in free iron accumulation in cardiomyocytes and mitochondria, further contributing to free radical generation, cell damage, and mitochondrial dysfunction [
19]. Anthracyclines also impair nitric oxide and endothelin-1 production, contributing to endothelial cell dysfunction and diminished cardiomyocyte survival [
20].
Genome wide association studies (GWAS) have contributed to the identification of possible genetic modifiers of risk for the development of anthracycline-induced cardiomyopathy; they are summarized in
Table 1. In a recent review, Magdy and colleagues summarized the single nucleotide polymorphisms (SNPs) that increase the risk of cardiotoxicity [
21]. For example, at the enzymatic level, the S427L variant of the
RARG gene increases topoisomerase IIβ expression and is associated with worse cardiac outcomes among pediatric cancer survivors [
22]. Variants in NAD(P)H oxidase subunits have been proposed to result in both acute and chronic forms of cardiotoxicity via ROS formation; [
23,
24,
25] Cascales et al. examined cardiac histology among recently deceased patients treated with anthracyclines and discovered a five-fold increased odds (95% CI: 1.59–16.43) of cardiac interstitial fibrosis in the presence of the rs1883112 SNP in the p40phox subunit of NAD(P)H oxidase [
26]. However, the rs4673 SNP in the p22phos subunit of NAD(P)H showed mixed results. While Cascales and colleagues noted a protective effect for those with the rs4673 SNP against myocardial fibrosis in the decedent samples (OR 0.11, 95% CI: 0.2–0.63), Wojnowski et al. found an association of this SNP with acute cardiotoxicity [
23,
26]. Moving from the enzymatic to the sarcomeric level, variants in the titin truncating gene (
TTN) have been found to occur at higher rates in patients with cardiotoxicity than in the general population [
27]. Garcia-Pavia subsequently confirmed the development of cardiomyopathy in
TTN variant mice treated with anthracyclines [
27].
Cardiotoxicity is also thought to arise from alterations in anthracycline transport and metabolism. In Magdy et al.’s review of the literature, they approximated that 45% of SNPs implicated in cardiotoxicity are associated with drug transport, which largely take the form of regulating the concentration of doxorubicin and its metabolites intracellularly [
21].
2.2. Non-Anthracycline Agents
Many non-anthracycline chemotherapy agents are thought to exert their cardiotoxic effects via oxidative stress as well, though at much lower rates than their anthracycline counterparts. For example, a recent review, Zhang and colleagues summarize studies that suggest cyclophosphamide and its metabolites have the potential to both increase ROS production and interfere with the antioxidant system [
32]. Cisplatin has been found to cause an accumulation of ROS, while anti-microtubule agents like vinblastine have been shown to decrease the activity of ROS scavenging enzymes [
32,
33].
Endothelial damage is thought to be a mechanism of cardiotoxicity mediated by non-anthracyclines as well. There is evidence that alkylators like cyclophosphamide directly damage endothelial cells, allowing for the extravasation of toxic metabolites and direct damage to myocytes [
34]. While cardiotoxicity from 5-fluorouracil (5FU) is rare, it can occur and take the form of acute coronary syndrome, which may be mediated by nitric oxide synthase (NOS) causing coronary artery spasm, endothelium-dependent vasoconstriction, and ultimately, ischemia [
35,
36]. Finally, thromboembolism formation may be promoted by agents like cisplatin and cyclophosphamide, similarly predisposing to ischemia [
34].
2.3. Radiation-Induced Cardiotoxicity
2.3.1. Mechanisms for Toxicity
Radiation therapy (RT) has improved oncologic outcomes for many pediatric cancers; however, RT to the chest is associated with a risk of cardiotoxicity. Radiation induces DNA damage, oxidative stress, endothelial cell senescence, and pro-inflammatory pathways. These changes may result in intimal thickening, fibrin deposition, lipid accumulation, and thrombosis [
37]. In the acute time period, pericarditis and myocarditis may be observed; however, their incidence is low with modern RT. Late effects, such as coronary artery disease (CAD), valvular disease, restrictive cardiomyopathy, and arrhythmias may become apparent years or decades after RT [
37,
38]. Importantly, the age at onset of late effects is variable and depends in part on the patient’s age when cancer therapy commenced. While the median age of onset of cardiac disease in those exposed to radiation and other cardiotoxic therapies is generally in young adulthood, myocardial infarctions, congestive heart failure, pericardial disease, and valvular abnormalities can occur in the childhood years [
5].
Other treatment and patient-specific factors increase the risk of radiation-associated cardiotoxicity. For example, the incidence of cardiotoxicity is higher in patients who received both anthracycline-based chemotherapy and RT compared to RT alone [
39,
40]. In addition, conventional risk factors, such as dyslipidemia, hypertension, and smoking, are independently associated with late cardiotoxicity [
41,
42,
43,
44]. Therefore, guidelines recommend regular screening for modifiable cardiac risk factors and the initiation of appropriate interventions in survivors who received RT to the chest [
45].
2.3.2. Dose-Toxicity Relationship
Radiation-specific factors that influence the risk of cardiotoxicity include the total radiation dose to the heart, the volume of the heart exposed, and the dose per fraction [
40,
41,
42,
43,
44,
46,
47] (
Table 2). Recent data from the Childhood Cancer Survivor Study demonstrated a significant association between mean heart dose (MHD) and multiple adverse cardiac outcomes (heart failure, CAD, valvular disease, arrhythmia) in multivariable models that accounted for sex, treatment decade, anthracycline dose, and relevant comorbidities [
41]. Importantly, this same study demonstrated that both the MHD and the risk of CAD in long-term survivors declined significantly over the study period. The decreased incidence of CAD with treatment decade was attenuated by adjustment for cardiac radiation exposure, suggesting that more modern RT is associated with a lower risk of CAD than historic RT [
41].
2.4. Targeted Cancer Therapies
Targeted chemotherapy is more widely used in pediatric cancer, as our understanding of specific mutations in cancer development and the immune regulation to keep tumors in check is advanced. These therapies include immune checkpoint inhibitors, tyrosine kinase inhibitors (TKIs), and proteasome inhibitors. While there is great optimism for their role in revolutionizing cancer therapy, they are not without side effects, including cardiotoxicity.
Immune checkpoint inhibitors work by blocking tumors’ attempts at evading the immune system, primarily by re-engaging T cell detection of tumors (this concept is further demonstrated in
Figure 2). Tumors learn to express cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death 1 (PD-1), and the ligand for PD-1 (PD-L1), all of which downregulate the immune response. Monoclonal antibodies against these receptors antagonize these downregulatory functions (effectively turning off the off switch) and allow the immune system to join in the fight against malignancy [
50]. Examples include ipilimumab against CTLA-4 and nivolumab and pembrolizumab against PD-1.
.5. Cellular Therapy and Hematopoietic Stem Cell Transplantation
Many childhood diseases, malignant and otherwise, are seeing improved survival rates as a result of allogenic or autologous bone marrow transplantation. The cardiotoxicity experienced in this population of children varies according to the risk factors they carry prior to transplant as well as their experiences post-transplant. Among patients transplanted for malignancies, many children are placed at a higher risk for developing cardiotoxicity due to pre-treatment with high doses of anthracyclines to induce a remission prior to their consolidative transplant [
61]. Patients transplanted for non-malignant conditions, such as sickle cell disease or thalassemia, often receive repeated red blood cell transfusions and are thus at higher risk for iron overload. Iron can deposit in the myocardium, increasing this population’s risk for eventual heart failure [
61]. Finally, malignant and non-malignant patients alike may receive total body irradiation as a part of their preparative regimen for bone marrow transplantation, increasing their risk for cardiotoxicity for the reasons mentioned previously [
61].
A number of factors that arise post-bone marrow transplantation increase the risk for cardiotoxicity as well. Patients are most often treated with calcineurin inhibitors (e.g., cyclosporin) as prophylaxis for graft versus host disease (GVHD). Calcineurin inhibitors are associated with systemic hypertension, and if blood pressures are not adequately managed, can increase the risk for development of heart failure [
61]. If GVHD does occur, it is often treated with corticosteroids, sometimes for long periods of time. The side effect profile of corticosteroids includes hypertension, hyperglycemia, and weight gain, which are all risk factors for heart disease [
61]. Finally, there is a higher incidence of metabolic syndrome in the post-transplant population, which compounds the risk factors that are already present [
61]. While development of heart failure as a consequence of these modifiable risk factors can take years or even decades to develop, the appropriate identification and treatment of these issues in the childhood years are critical to mitigate future cardiovascular risk and improve longer term outcomes.
Cellular therapy is an evolving field for treatment of childhood cancers. Most promising thus far is the development of chimeric antigen receptor (CAR) T cells. To engineer a CAR-T cell, a patient’s T cells are collected via leukapheresis, injected with a genetically engineered receptor to target antigens on the surface of tumor cells, expanded, and infused back into the patient. This was first used in pediatric pre-B acute lymphoblastic leukemia (ALL), where CD-19 on the surface of B cells was targeted by CAR-T cells [
62]. Patients treated with CAR-T therapy are at risk for cardiotoxicity both because of treatment they may have seen in the past (e.g., anthracyclines) in addition to acute risks associated with the CAR-T cells, most notably cytokine release syndrome (CRS).
CRS is a phenomenon in which the infused CAR-T cells stimulate both the innate and adaptive immune system, releasing supraphysiologic levels of cytokines leading to systemic inflammation [
63]. CRS ranges from mild to severe, with signs and symptoms ranging from fever and tachycardia to hypoxia, hypotension, and, in the worst case, organ failure and death. IL-6 is an important mediator of CRS and is released by antigen presenting cells (APCs) and activated endothelial cells [
50]. The dysfunction of endothelial cells then contributes to vascular leak and disruption of the blood brain barrier, leading to CAR-T cell induced neurotoxicity (i.e., immune effector cell associated neurotoxicity/ICANS). IL-6 is thought to be secreted at the same time CAR-T cells are recognizing antigenic targets on tumor cells, not as a direct result of CAR-T cells engaging APCs or endothelial cells [
50]. The role of IL-6 in CRS is further supported by the rapid improvement seen with the initiation of tociluzimab, a monoclonal antibody against the IL-6 receptor [
64].
Acute cardiotoxicity in the setting of CAR-T cell therapy occurs almost exclusively in the setting of CRS and most closely resembles cardiomyopathy associated with sepsis [
50]. IL-6 is largely to blame for cardiotoxicity in this context, which is consistent with previous work showing this cytokine to be implicated in myocardial depression in both inflammatory and infectious states [
65]. Supraphysiologic levels of this and other pro-inflammatory cytokines can lead to tachycardia, hypotension, troponin elevation, reduced left ventricular ejection fraction, pulmonary edema, and cardiogenic shock [
50].
Finally, metabolic derangements can play a role in CAR-T cell associated cardiotoxicity. When CAR-T cells engage target antigens on the surface of tumor cells and induce cell lysis, intracellular electrolytes such as potassium, phosphate, calcium, and uric acid spill into the extracellular space in a phenomenon known as tumor lysis syndrome. These metabolic derangements may lead to arrythmia and renal failure if left unchecked [
50].
This entry is adapted from the peer-reviewed paper 10.3390/children8090829