Animal proteins are good sources of protein for human, due to the composition of necessary amino acids. The quality of food depends significantly on the properties of protein inside, especially the gelation, transportation, and antimicrobial properties. Interestingly, various kinds of molecules co-exist with proteins in foodstuff, and the interactions between these can significantly affect the food quality.
- protein
- animal food
- food quality
- interactions
1. Introduction
Animal foods are good source of “high quality” protein for humans, especially lean meat, milk, eggs, and fishery products [1]. Diet composition improvement represents a key factor to enhance the health status and welfare of animals [2,3] as well as to enhance productivity and performance in livestock [4,5]. Proteins have been widely used as food components in the food industry due to their nutritional value and functional properties [6]. In the past several years, chemical modifications such as oxidation and glycosylation have been developed to improve the functional properties [7]. It is worth mentioning that gelation, oxidation, and assembly of proteins occurred which may affect the texture, nutrition, and sensory properties of food during food processing and storage [8]. Recent research has mainly focused on the improvement of gelation, inhibition of oxidation and assembly of proteins. Taking advantage of the antioxidant properties of polyphenols, the protein-polyphenol interactions have been reported to help inhibit or slow down the oxidation of protein from meat [9], and further affect the gelation process.
The micro- and macroscopic structures of food determine the behaviors of food. Proteins, especially myofibrillar protein in meat, contribute most to the formation of three-dimensional network structure in processed meat products, which will help to hold water inside and confer meat with a “juicy” taste. On the other hand, protein gelation also contributes to the quality of dairy products [10]. More interestingly, the gelation process can be improved by other molecules such as polysaccharides and polyphenols. Meanwhile, proteins in milk such as bovine serum albumin and lactoferrin play crucial roles in nutrient transportation [11]. The interactions between proteins and substances from environmental pollution or molecules produced during food processing may affect the transporting efficiencies to some extent [12].
In addition to the interactions between protein and small molecules, the antibacterial activities and enzymatic activities of proteins or enzymes also affect the product quality especially the storage stability and shelf-life. Therefore, it is imperative to summarize the roles of proteins from animal sources in food quality, and the effects of interactions between proteins and small molecules on food texture, and sensory and nutritional properties. As for the selection of research papers, we have analyzed the main proteins/enzymes affecting food qualities in each kind of food, which are myofibrillar protein, hemoglobin, whey protein, bovine serum albumin, casein, lactoferrin, lysozyme, ovalbumin, collagen, polyphenol oxidase, and ferritin ( Table 1 ). Thus, we focused on these proteins and the interactions affecting quality. This review will not only provide guidelines for food processing to improve the quality of food products, but also make it possible to increase stability by regulating the properties of proteins or enzymes.
| Food Sources | Main Proteins | Relationships with Product Quality | References |
|---|---|---|---|
| Meat | Myofibrillar protein | Form heat-induced gel Related to the water retention, chewiness and juiciness of meat products. |
[13,14] |
| Sarcoplasmic protein | Related to the color of meat products. Influence gel properties, such as water holding capacity. Indirectly affects meat tenderness. | [15,16] | |
| Connective tissue | Affect the texture, tenderness and water holding capacity of meat products. | [17] | |
| Hemoglobin | Soluble, foaming, emulsifying, colorant, and nutritional supplement. | [18,19] | |
| Plasma protein | Foaming, solubility, emulsifying, gelling properties, and fat binder. | [18,20] | |
| Milk | Whey protein | Emulsification, foaming, gelling, water binding properties. Used as thickener, stabilizers, and fat substitutes in yogurt. |
[21,22,23] |
| Bovine serum albumin | Physiological model protein, foaming, emulsifying, gelling properties. | [24] | |
| Casein | Solubility, emulsifying, gelling, foaming, stability. Can be used to make food films. | [25,26,27] | |
| Lactoferrin | Biological activities: iron metabolism regulation, immune regulation, antibacterial, antitumor, anti-inflammatory. Can be used as iron solubilizer. | [28,29] | |
| Egg | Egg white proteins | Gelling, water holding capacity, foaming and emulsifying properties. Can be used as thickener to give the product good taste and texture. Enhance the nutritional level. Can be used as food packaging film. | [30,31] |
| Egg yolk proteins | Gelling, emulsification, film formation. Can be used as gelata, adhesive or color former to improve the nutritional value, flavor and taste of the product. |
[32,33] | |
| Lysozyme | Antibacterial activity. Extend the shelf life of food. |
[34] | |
| Fishery products | Collagen | Used as emulsifier, foaming agent, stabilizer and nutrients in the product. | [35,36] |
| Ferritin | Can be used as iron supplement. | [37] | |
| Polyphenol oxidase | Cause melanosis of crustaceans. | [38] | |
| Transglutaminase | Improve functional properties of proteins, such as gelation. | [39,40] | |
| Cathepsins | Promoting degradation of myofibrillar protein. | [41] |
2. Proteins in Meat
At present, a variety of substances are used to improve the structure of MP gel to produce meat products with better quality and taste ( Figure 1 ). Transglutaminase is a substance that promotes the widespread use of meat protein gels. A non-disulfide covalent bond is formed by transglutaminase between glutamine residues and lysine residues within and between proteins to cross-link protein molecules, thereby improving the properties of protein gels [45,46]. Moreover, some plant proteins which can cross-link with MP, form more stable gels by hydrophobic interactions and disulfide bonds, and improve the cooking yield of MP gels under the induction of glutamic amide transaminase, have been applied to improve the texture and sensory properties of products in meat processing [47,48,49,50,51,52].
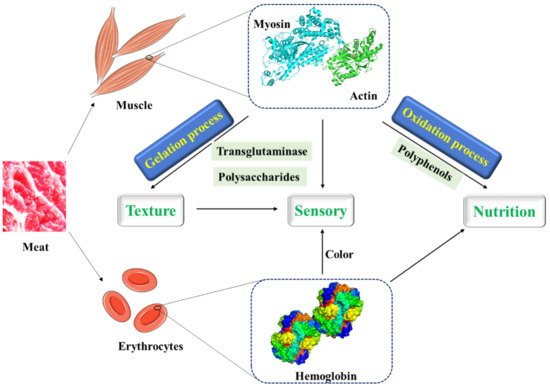
Meat is a very efficient deliverer of protein. In addition to fresh meat, meat is usually further processed to improve the sensory quality and to extend their shelf life. During food processing, large amounts of polysaccharides such as κ-carrageenan and flaxseed gum have been used in comminuted meat products to improve their functional properties such as emulsification, fat, and water binding capacity, while improving their texture and appearance.
In low-fat meat products, polysaccharides acting as water binders affect the thermal transition temperatures of meat proteins by interacting with meat protein. The electrostatic force in nature is the principal force responsible for these interactions. Meanwhile, by studying the thermal properties, dynamic rheological properties, texture and microstructure of salt-soluble meat protein and flaxseed gum, it was found that the interaction between salt-soluble meat protein and flaxseed gum occurred, and the main force for the formation and stabilization of protein-polysaccharide gels seemed to be electrostatic force [71,72]. The gel formed by flaxseed gum is thermoreversible, which is significantly related to the flavor release, perception of “juiciness” and oral retention times of meat products [71].
In addition, κ-carrageenan as a linear sulphated polysaccharide is usually added to canned meats and pet foods as a gelatinizer. The thermal reversible gel properties of carrageenan affect the function of meat products, which are important to the sensory quality of meat. Carrageenan is dissolved in the whole meat during hot processing and solidified during cooling, which can improve water retention, texture, and consistency of comminuted meat products [73]. However, instead of chemically interacting with meat proteins, carrageenans increased the water-holding capacity of meat emulsions by physically holding water in the interstitial spaces of the protein–gel network. In practical application, other factors may affect the properties of meat protein as well. During the cooking process, changes in quaternary, tertiary, and secondary structure of proteins occur, which may further induce the changes in intra/intermolecular interactions. In sodium-reduced chicken breast myofibrillar protein gel, high pressure processing and calcium have been used to increase the water holding capacity [74]. Moreover, in emulsified meat products such as sausages, phosphate elimination affected the gelling characteristics of the products, and egg or pea addition remediated the quality loss [75]. More importantly, two transglutaminase enzymes were used in the restructured beef steaks to create covalent linking between meat proteins and improve the quality [76].
3. Proteins in Milk
Whey proteins are milk proteins (mainly β-lactoglobulin and α-lactalbumin) that are widely used in food design due to a variety of functionalities, including: water binding, gelation, emulsification, and foaming [77]. In food processing, whey proteins are usually denatured due to heat treatments and thereby they aggregate, either in self-aggregation or with other food particles [78,79]. Through extensive research, it has been shown that the interactions between whey proteins and small molecules can change their molecular structures and the physico-chemical properties [79], supporting their applications in food.
Most chitosans, which have been shown to effectively reduce the cholesterol blood level in animals and humans by strongly binding to the negatively charged bile acids, have the ability to interact with whey protein in milk [80]. At low pH, a weak carbohydrate-protein interaction is formed between chitosan and whey protein isolate, both of which are positively charged. When the pH is raised to 6.0, where the protein should be negatively charged, a much stronger interaction occurs due to electrostatic attraction between them, indicating that pH plays an overwhelming role in this complex system [10]. Therefore, these interactions can help separating whey protein during diary processing. Meanwhile, as a gelled agent, carrageenan is commonly used in the production of milk-based desserts. The addition of κ-carrageenan to whey protein-based desserts affects gel formation and caused an even higher storage modulus than milk-based desserts, possibly due to the interactions between whey proteins and κ-carrageenan at different pH values. At low pH, especially after heating, the whey protein will be exposed to a large number of hydrophobic groups to form large aggregates, which is the result of strong interactions with κ-carrageenan. In contrast, electrostatic repulsions between polysaccharide molecules and the negatively charged protein occurred at high pH. Therefore, optimal conditions help the production of highly digestible, light-colored whey protein components. In addition, phenolic-rich foods such as coffee and fruit juices are often consumed together with milk. Milk protein can affect the bioavailability of polyphenols through their interactions. Consistent with this idea, it has been shown that the phenolic acids (such as chlorogenic acid, ferulic acid, caffeic acid, and coumalic acid) can not only quench the fluorescence of β-lactoglobulin and α-lactalbumin by static quenching but also significantly affect the secondary structure of β-lactoglobulin and α-lactalbumin. More specifically, phenolic acids were proved to interact with the C=O and C-N groups of the structural subunits of whey protein [81]. As a consequence, the interaction between whey protein and other molecules not only affects the application of whey protein in the food industry but also the bioavailability ( Figure 2 ).
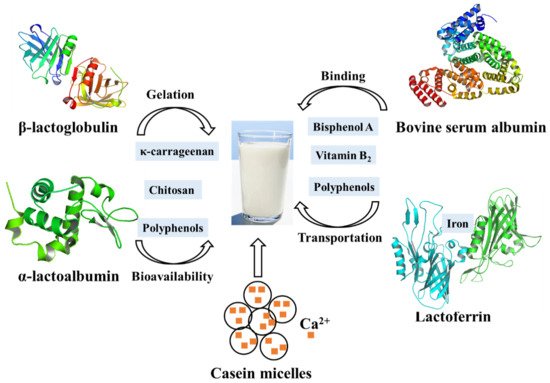
Caseins, the major proteins in milk, are a family of related phosphoproteins and consist of submicelles which in turn are aggregates of three calcium-insoluble proteins (αS1, αS2 and β-casein) and one calcium-soluble protein κ-casein [89]. The calcium-insoluble caseins are usually considered to be located predominantly within the micelles, while κ-casein is thought to coat the micelle, serving to stabilize the structure [90]. Additionally, the calcium-insoluble caseins are highly phosphorylated, and they interact with calcium phosphate interacts through their phosphate groups. In this way, the casein micelles carry a large number of (usually highly insoluble) calcium phosphate into milk and keep it suspended. However, the removal of subcritical amounts of Ca 2+ from casein micelles with EDTA releases soluble casein, suggesting that Ca 2+ removal initially dissociates weakly bound caseins from the micelle without destroying the micelle size determining framework. Generally, the minerals and caseins in milk are in dynamic equilibrium between the micellar (pelleted components) and serum phases (non-pelleted components) [91]. Temperature, pH, and mineral salts or calcium chelators can affect the partitioning of the components [91]. Studies of calcium-chelating agents showed that the amount of colloidal calcium phosphate removed from casein micelles limited the reversibility of calcium phosphate micelle dissolution and formation. Furthermore, addition of casein to milk has been proved to increase the Ca absorption in young rats [92].
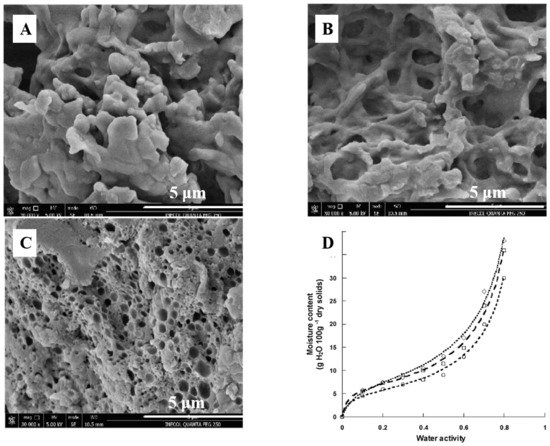
On the other hand, casein has a wide range of uses in food industry, especially in cheese. The milk salts, Ca 2+ and (PO 4) 3− , play an important role in the rennet coagulation of milk and in the structure and buffering of cheese by the neutralization of negatively charged residues on casein and aggregation of renneted micelles [93]. Addition of low concentrations of Ca 2+ also increases gel firmness, which is important for the cheese texture. Moreover, the functional properties of casein can be changed by modification. Casein modified by freezing or ultrasound exhibited better emulsifying capacity and water absorption capacity, helping to extend the shelf life of the products ( Figure 3 ) [94]. The partial replacement of casein in cheese by modified caseins can improve the microbial stability and shelf life of the cheeses. More interestingly, the interactions between casein and tannic acid have been further applied in food packaging, the results of which suggest that tannic acid is an effective crosslinking agent for casein protein, and can improve the water resistance of film [95].
4. Proteins in Egg
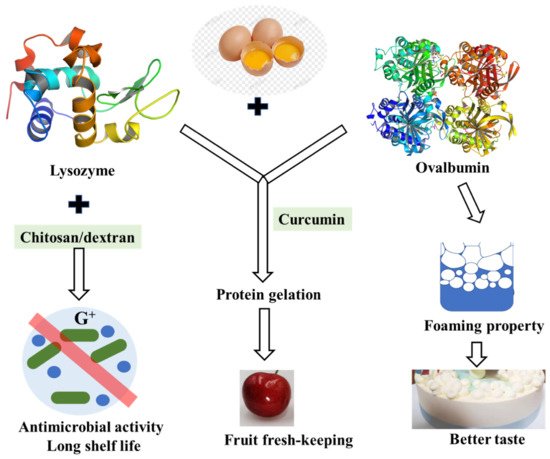
As one of the least expensive forms of protein, fresh shell eggs are among the most nutritious daily foods. However, shell eggs’ internal quality deteriorates, and bacteria grow easily during storage. Egg-white lysozyme is an important component in the prevention of bacterial growth in hen eggs and is usually used as a preservative to inhibit the growth of deleterious organisms thus prolonging shelf life [97]. Lysozyme is a kind of enzyme that lyses the cell walls of certain Gram-positive bacteria as it splits the bond between N-acetylmuramic acid and N-acetylglucosamine of the peptidoglycan in the bacterial cell wall [98]. Despite the direct bacteriolytic action, it is reported that lysozyme had many other biological functions such as antiviral action to inactivate certain viruses by forming an insoluble complex, anti-inflammatory and antihistaminic actions, potentiation of antibiotic effects, and so on. Nevertheless, the peptidoglycan envelope of gram-negative bacteria is known to consist mainly of lipopolysaccharide (LPS) and is resistant to natural lysozyme, and lysozyme was not suitable for general antimicrobial use due to its limited lytic spectrum. However, it is not suitable for general antibacterial use due to its limited lysozyme spectrum. When lysozyme is used with other substances such as chitosan or glucan, its antibacterial spectrum may be enhanced [99]. Research on lysozyme-dextran conjugate prepared by Maillard reaction showed that it has obvious antimicrobial activity against both Gram-negative and Gram-positive bacteria. The antimicrobial activity of the lysozyme-dextran conjugate exhibits stable lysozyme antimicrobial activity for Gram-negative bacteria and excellent emulsifying properties to solubilize the outer membrane [100].
Furthermore, lysozyme–chitosan coating solutions could be effectively produced for use in preservation, extending the shelf life of eggs by delaying the loss of interior quality during storage [99]. Developing different perishable food coatings with different antioxidant and antimicrobial effects is the goal of future work.
Ovalbumin is the main protein in egg white with good foaming, emulsifying, and gelation. It is found that the interaction between lysozyme and ovalbumin occurred in a chimeric mode, which may be caused by hydrophobic bonds [101]. The interaction between them is reversible, forming a homeostasis. Although this interaction reduced the bacteriolytic activity of lysozyme, the foaming properties and foam stability of egg white protein were decreased without lysozyme [102] ( Figure 4 ). The relationship between egg white proteins remains to be further studied.
Egg albumin is rich in disulfide bonds and thiol groups, and thin films can be formed by alkali and heat treatment [103]. Compared with commercial polylactic acid (PLA) film, egg white protein (EWP) film, which is produced by an extrusion and rolling process, has higher water permeability, rigidity, heat resistance, and oxygen barrier, which can be used as part of semi-solid and solid food packaging materials [104]. Huang [105] mixed egg white protein and κ-carrageenan to prepare edible composite films, and found that, with the increase of egg white protein content, the disorder degree of the composite film increased, the elongation at break and light transmittance increased, but the oxygen permeability, water vapor permeability and water soluble decreased. Compared with the commercial film, EWP/κ-C composite film can effectively delay the oil rancidity. Tiimob [106] found that the addition of egg white protein into poly butylene adipate-co-terephthalate (PBAT)/polylactic acid (PLA) blends could make the resulting film have a certain antibacterial effect, showing the potential of egg white for manufacturing food packaging materials. It is noteworthy that, in the production of oat noodles, wheat noodles and pasta, the addition of egg white can lower the cooking loss and increase the springinesss and resilience by influencing the water absorption and texture [31].
This entry is adapted from the peer-reviewed paper 10.3390/foods10091988
