Acquiring the recommended daily allowance of vitamins is crucial for maintaining homeostatic balance in humans and other animals. A deficiency in or dysregulation of vitamins adversely affects the neuronal metabolism, which may lead to neurodegenerative diseases. In this article, we discuss how novel vitamin-based approaches aid in attenuating abnormal neuronal functioning in neurodegeneration-based brain diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, Amyotrophic lateral sclerosis, and Prion disease. Vitamins show their therapeutic activity in Parkinson’s disease by antioxidative and anti-inflammatory activity. In addition, different water- and lipid-soluble vitamins have also prevented amyloid beta and tau pathology. On the other hand, some results also show no correlation between vitamin action and the prevention of neurodegenerative diseases. Some vitamins also exhibit toxic activity too.
- vitamins
- neurodegenerative disease
- Parkinson’s disease
- Alzheimer’s disease
- Huntington disease
- Prion disease
1. Introduction
2. The Role of Vitamins in Neurodegenerative Disease
2.1. Vitamins in Parkinson’s Disease
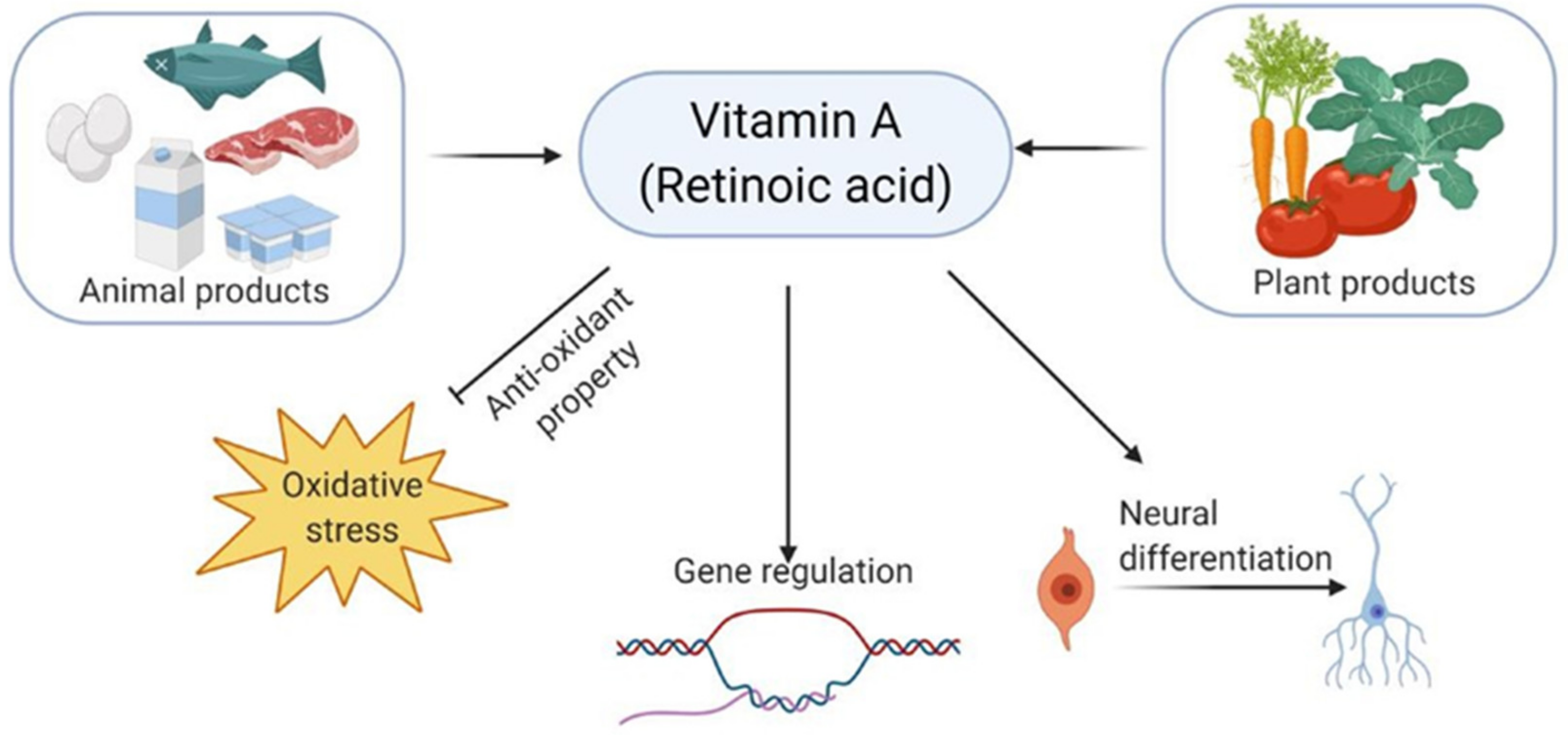
2.2. Vitamins in Alzheimer’s Disease
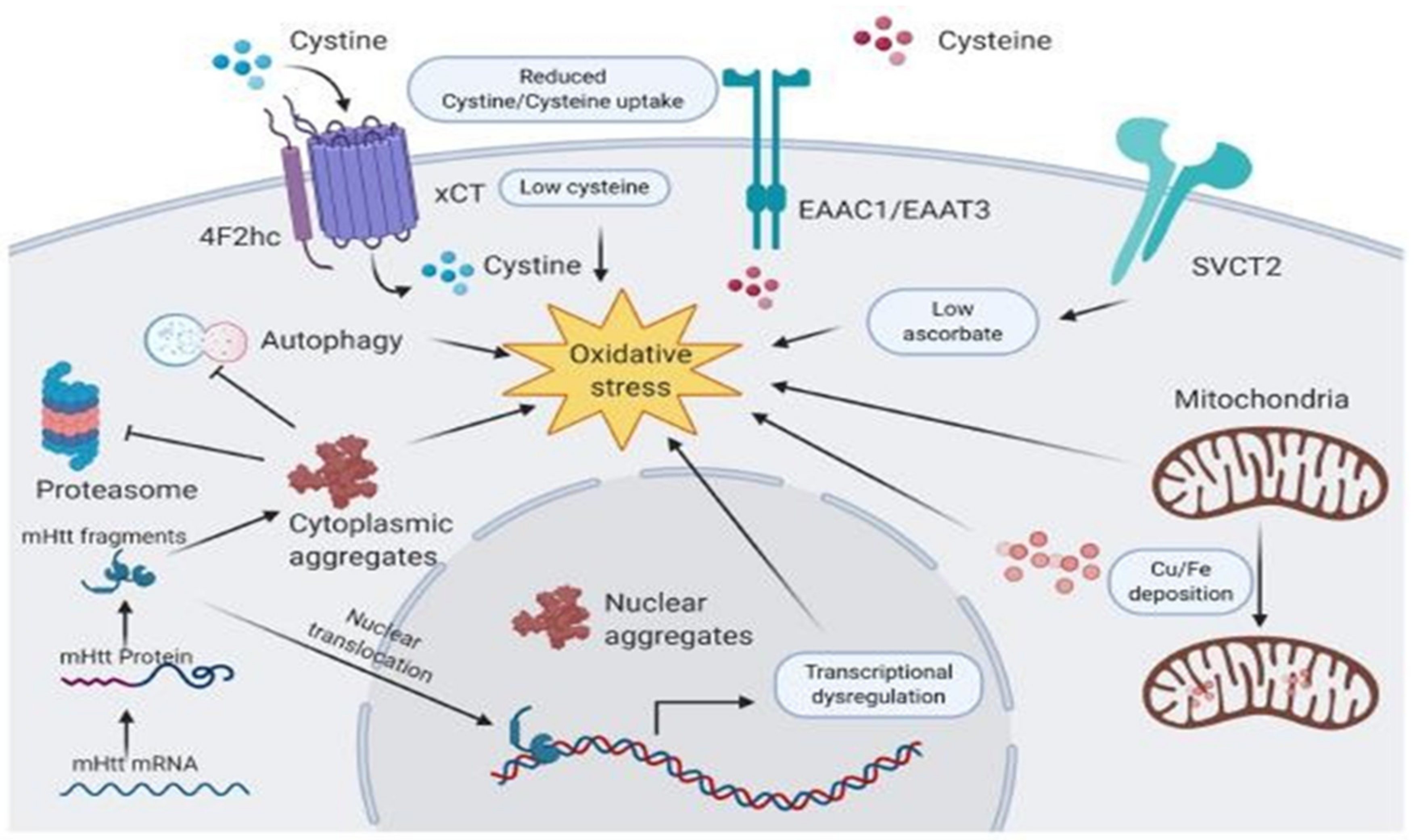
2.3. Vitamins in Huntington’s Disease
2.4. Vitamins in Multiple Sclerosis
2.5. Vitamins in Amyotrophic Lateral Sclerosis
2.6. Vitamins in Prion Disease
2.7. Vitamins in Age-Related Macular Degeneration
3. Conclusions and Future Prospective
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines9101284
References
- Darnton-Hill, I. Public health aspects in the prevention and control of vitamin deficiencies. Curr. Dev. Nutr. 2019, 3, nzz075.
- Shenkin, A. Micronutrients in health and disease. Postgrad. Med. J. 2006, 82, 559–567.
- Woteki, C.E.; Thomas, P.R. In eat for life: The food and nutrition board’s guide to reducing your risk of chronic disease. Clin. Nutr. Insight 1993, 19, 7.
- Heaney, R.P. The nutrient problem. Nutr. Rev. 2012, 70, 165–169.
- Shao, A.; Drewnowski, A.; Willcox, D.C.; Kramer, L.; Lausted, C.; Eggersdorfer, M.; Mathers, J.; Bell, J.D.; Randolph, R.K.; Witkamp, R.; et al. Optimal nutrition and the ever- changing dietary landscape: A conference report. Eur. J. Nutr. 2017, 56, 1–21.
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 71.
- Sies, H.; Stahl, W.; Sundquist, A.R. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann. N. Y. Acad. Sci. 1992, 669, 7–20.
- Albahrani, A.A.; Greaves, R.F. Fat-soluble vitamins: Clinical indications and current challenges for chromatographic measurement. Clin. Biochem. Rev. 2016, 37, 27.
- Bruno, E.J.; Ziegenfuss, T.N. Water-soluble vitamins: Research update. Curr. Sports Med. Rep. 2005, 4, 207–213.
- Rai, S.N.; Birla, H.; Singh, S.S.; Zahra, W.; Patil, R.R.; Jadhav, J.P.; Gedda, M.R.; Singh, S.P. Mucuna pruriens protects against MPTP Intoxicated neuroinflammation in Parkinson’s disease through NF-κB/pAKT signaling pathways. Front. Aging Neurosci. 2017, 9, 421.
- Rai, S.N.; Birla, H.; Zahra, W.; Singh, S.S.; Singh, S.P. Immunomodulation of Parkinson’s disease using Mucuna pruriens (Mp). J. Chem. Neuroanat. 2017, 85, 27–35.
- Rai, S.N.; Zahra, W.; Singh, S.S.; Birla, H.; Keswani, C.; Dilnashin, H.; Rathore, A.S.; Singh, R.; Singh, R.K.; Singh, S.P. Anti-inflammatory activity of ursolic acid in MPTP-induced parkinsonian mouse model. Neurotox. Res. 2019, 36, 452–462.
- Rai, S.N.; Chaturvedi, V.K.; Singh, P.; Singh, B.K.; Singh, M.P. Mucuna pruriens in Parkinson’s and in some other diseases: Recent advancement and future prospective. 3 Biotech 2020, 10, 522.
- Rai, S.N.; Singh, P.; Varshney, R.; Chaturvedi, V.K.; Vamanu, E.; Singh, M.P.; Singh, B.K. Promising drug targets and associated therapeutic interventions in Parkinson’s disease. Neural Regen. Res. 2021, 16, 1730–1739.
- Rodriguez, M.; Rodriguez-Sabate, C.; Morales, I.; Sanchez, A.; Sabate, M. Parkinson’s disease as a result of aging. Aging Cell 2015, 14, 293–308.
- Goldenberg, M.M. Medical management of Parkinson’s disease. Pharm. Ther. 2008, 33, 590.
- Elkouzi, A.; Vedam-Mai, V.; Eisinger, R.S.; Okun, M.S. Emerging therapies in Parkinson disease—Repurposed drugs and new approaches. Nat. Rev. Neurol. 2019, 15, 204–223.
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of diet and nutritional supplements in Parkinson’s disease progression. Oxid. Med. Cell. Longev. 2017, 2017, 1–9.
- Ciulla, M.; Marinelli, L.; Cacciatore, I.; Stefano, A.D. Role of dietary supplements in the management of Parkinson’s disease. Biomolecules 2019, 9, 271.
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of vitamin A in the immune system. J. Clin. Med. 2018, 7, 258.
- Dias, V.; Junn, E.; Mouradian, M. The role of oxidative stress in Parkinson’s disease. J. Park. Dis. 2013, 3, 461–491.
- Gilbert, C. What is vitamin A and why do we need it? Community Eye Health 2013, 26, 65.
- Burri, B.J.; Chang, J.S.; Neidlinger, T.R. beta-Cryptoxanthin- and alpha-carotene-rich foods have greater apparent bioavailability than beta-carotene-rich foods in Western diets. Br. J. Nutr. 2011, 105, 212–219.
- Al Tanoury, Z.; Piskunov, A.; Rochette-Egly, C. Vitamin A and retinoid signaling: Genomic and nongenomic effects. J. Lipid Res. 2013, 54, 1761–1775.
- Janesick, A.; Wu, S.C.; Blumberg, B. Retinoic acid signaling and neuronal differentiation. Cell. Mol. Life Sci. 2015, 72, 1559–1576.
- Tafti, M.; Ghyselinck, N.B. Functional implication of the vitamin A signaling pathway in the brain. Arch. Neurol. 2007, 64, 1706–1711.
- Dong, R.; Wang, H.; Ye, J.; Wang, M.; Bi, Y. Publication trends for Alzheimer’s disease worldwide and in China: A 30-year bibliometric analysis. Front. Human Neurosci. 2019, 13, 259.
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189.
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegen. 2019, 14, 1–18.
- Lacosta, A.-M.; Insua, D.; Badi, H.; Pesini, P.; Sarasa, M. Neurofibrillary tangles of Aβ x- 40 in Alzheimer’s disease brains. J. Alzheimers Dis. 2017, 58, 661–667.
- Castellani, R.J.; Lee, H.-G.; Zhu, X.; Nunomura, A.; Perry, G.; Smith, M.A. Neuropathology of Alzheimer disease: Pathognomonic but not pathogenic. Acta Neuropathol. 2006, 111, 503–509.
- Tönnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alz. Dis. 2017, 57, 1105–1121.
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162.
- Caron, N.S.; Wright, G.E.B.; Hayden, M.R. Huntington Disease; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; GeneReviews: Seattle, DC, USA, 1993. Available online: http://www.ncbi.nlm.nih.gov/books/nbk1305/ (accessed on 17 September 2021).
- Dayalu, P.; Albin, R.L. Huntington disease: Pathogenesis and treatment. Neurol. Clin. 2015, 33, 101–114.
- Chel, V.G.; Ooms, M.E.; van der Bent, J.; Veldkamp, F.; Roos, R.A.; Achterberg, W.P.; Lips, P. High prevalence of vitamin D deficiency and insufficiency in patients with manifest Huntington disease: An explorative study. Dermato Endocrinol. 2013, 5, 348–351.
- Roos, R. Huntington’s disease: A clinical review. Orphanet J. Rare Dis. 2010, 5, 40.
- Patassini, S.; Begley, P.; Xu, J.; Church, S.J.; Kureishy, N.; Reid, S.J.; Waldvogel, H.J.; Faull, R.L.; Snell, R.G.; Unwin, R.D. Cerebral vitamin B5 (D-Pantothenic Acid) deficiency as a potential cause of metabolic perturbation and neurodegeneration in Huntington’s disease. Metabolites 2019, 9, 113.
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74.
- Johri, A.; Beal, M.F. Antioxidants in Huntington’s disease. Biochim. Biophys. Acta 2012, 1822, 664–674.
- Fitzner, D.; Simons, M. Chronic progressive multiple sclerosis-pathogenesis of neurodegeneration and therapeutic strategies. Curr. Neuropharmacol. 2010, 8, 305–315.
- Correale, J.; Marrodan, M.; Ysrraelit, M.C. Mechanisms of neurodegeneration and axonal dysfunction in progressive multiple sclerosis. Biomedicines 2019, 7, 14.
- Miljković, D.; Spasojević, I. Multiple sclerosis: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2013, 19, 2286–2334.
- Serra, A.; Chisari, C.G.; Matta, M. Eye movement abnormalities in multiple sclerosis: Pathogenesis, modeling, and treatment. Front. Neurol. 2018, 9, 31.
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40.
- Adzemovic, M.Z.; Zeitelhofer, M.; Hochmeister, S.; Gustafsson, S.A.; Jagodic, M. Efficacy of vitamin D in treating multiple sclerosis-like neuroinflammation depends on developmental stage. Exp. Neurol. 2013, 249, 39–48.
- Munger, K.L.; Åivo, J.; Hongell, K.; Soilu-Hänninen, M.; Surcel, H.-M.; Ascherio, A. Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the Finnish maternity cohort. JAMA Neurol. 2016, 73, 515–519.
- Smolders, J.; Damoiseaux, J.; Menheere, P.; Hupperts, R. Vitamin D as an immune modulator in multiple sclerosis, a review. J. Neuroimmun. 2008, 194, 7–17.
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and multiple sclerosis: A comprehensive review. Neurol. Ther. 2018, 7, 59–85.
- Ascherio, A.; Weisskopf, M.G.; O’Reilly, E.J.; Jacobs, E.J.; McCullough, M.L.; Calle, E.E.; Cudkowicz, M.; Thun, M.J. Vitamin E intake and risk of amyotrophic lateral sclerosis. Ann. Neurol. 2005, 57, 104–110.
- Niino, M.; Fukazawa, T.; Kikuchi, S.; Sasaki, H. Therapeutic potential of vitamin D for multiple sclerosis. Curr. Med. Chem. 2008, 15, 499–505.
- Ramagopalan, S.V.; Dobson, R.; Meier, U.C.; Giovannoni, G. Multiple sclerosis: Risk factors, prodromes, and potential causal pathways. Lancet Neurol. 2010, 9, 727–739.
- Sandberg, L.; Biström, M.; Salzer, J.; Vågberg, M.; Svenningsson, A.; Sundström, P. Vitamin D and axonal injury in multiple sclerosis. Mult. Scler. J. 2016, 22, 1027–1031.
- Muller, T.; Lohse, L.; Blodau, A.; Frommholz, K. Vitamin D rise enhances blood perfusion in patients with multiple sclerosis. J. Neural Transm. 2019, 126, 1631–1636.
- Perga, S.; Albo, A.G.; Lis, K.; Minari, N.; Falvo, S.; Marnetto, F.; Caldano, M.; Reviglione, R.; Berchialla, P.; Capobianco, M.A. Vitamin D binding protein isoforms and apolipoprotein E in cerebrospinal fluid as prognostic biomarkers of multiple sclerosis. PLoS ONE 2015, 10, e0129291.
- Disanto, G.; Ramagopalan, S.V.; Para, A.E.; Handunnetthi, L. The emerging role of vitamin D binding protein in multiple sclerosis. J. Neurol. 2011, 258, 353–358.
- Smolders, J.; Peelen, E.; Thewissen, M.; Menheere, P.; Damoiseaux, J.; Hupperts, R. Circulating vitamin D binding protein levels are not associated with relapses or with vitamin D status in multiple sclerosis. Mult. Scler. J. 2014, 20, 433–437.
- Talbot, K. Motor neuron disease: The bare essentials. Pract. Neurol. 2009, 9, 303–309.
- Martin, S.; Al Khleifat, A.; Al-Chalabi, A. What causes amyotrophic lateral sclerosis? F1000Research 2017, 6, 371.
- Zarei, S.; Carr, K.; Reiley, L.; Diaz, K.; Guerra, O.; Altamirano, P.F.; Pagani, W.; Lodin, D.; Orozco, G.; Chinea, A. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 2015, 6, 171.
- Luong, K.V.Q.; Nguyễn, L.T.H. Roles of vitamin D in amyotrophic lateral sclerosis: Possible genetic and cellular signaling mechanisms. Mol. Brain 2013, 6, 16.
- Cortese, R.; D’Errico, E.; Introna, A.; Schirosi, G.; Scarafino, A.; Distaso, E.; Nazzaro, P.; Zoccolella, S.; Simone, I. Vitamin D levels in serum of amyotrophic lateral sclerosis patients. (P2. 069). AAN Enterp. 2015, 84, 14S.
- Camu, W.; Tremblier, B.; Plassot, C.; Alphandery, S.; Salsac, C.; Pageot, N.; Juntas-Morales, R.; Scamps, F.; Daures, J.P.; Raoul, C. Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis. Neurobiol. Aging 2014, 35, 1198–1205.
- Duong, S.; Patel, T.; Chang, F. Dementia: What pharmacists need to know. Can. Pharm. J./Rev. Pharm. Can. 2017, 150, 118–129.
- Cunningham, E.L.; McGuinness, B.; Herron, B.; Passmore, A.P. Dementia. Ulster Med. J. 2015, 84, 79–87.
- Gibson, G.E.; Hirsch, J.A.; Fonzetti, P.; Jordon, B.D.; Cirio, R.T.; Elder, J. Vitamin B1 (thiamine) and dementia. Ann. N. Y. Acad. Sci. 2016, 1367, 21.
- Blundo, C.; Marin, D.; Ricci, M. Vitamin B12 deficiency associated with symptoms of frontotemporal dementia. Neurol. Sci. 2011, 32, 101–105.
- Malaguarnera, M.; Ferri, R.; Bella, R.; Alagona, G.; Carnemolla, A.; Pennisi, G. Homocysteine, vitamin B12 and folate in vascular dementia and in Alzheimer disease. Clin. Chem. Lab. Med. 2004, 42, 1032–1035.
- Hegyi, J.; Schwartz, R.A.; Hegyi, V. Pellagra: Dermatitis, dementia, and diarrhea. Int. J. Dermatol. 2004, 43, 1–5.
- Zhang, X.Y.; Xu, Z.P.; Wang, W.; Cao, J.B.; Fu, Q.; Zhao, W.X.; Li, Y.; Huo, X.L.; Zhang, L.M.; Li, Y.F.; et al. Vitamin C alleviates LPS-induced cognitive impairment in mice by suppressing neuroinflammation and oxidative stress. Int. Immunopharmacol. 2018, 65, 438–447.
- Olajide, O.J.; Yawson, E.O.; Gbadamosi, I.T.; Arogundade, T.T.; Lambe, E.; Obasi, K.; Lawal, I.T.; Ibrahim, A.; Ogunrinola, K.Y. Ascorbic acid ameliorates behavioural deficits and neuropathological alterations in rat model of Alzheimer’s disease. Environ. Toxicol. Pharmacol. 2017, 50, 200–211.
- Sil, S.; Ghosh, T.; Gupta, P.; Ghosh, R.; Kabir, S.N.; Roy, A. Dual Role of Vitamin C on the Neuroinflammation Mediated Neurodegeneration and Memory Impairments in Colchicine Induced Rat Model of Alzheimer Disease. J. Mol. Neurosci. 2016, 60, 421–435.
- Yamini, P.; Ray, R.S.; Chopra, K. Vitamin D3 attenuates cognitive deficits and neuroinflammatory responses in ICV-STZ induced sporadic Alzheimer’s disease. Inflammopharmacology 2018, 26, 39–55.
- Briones, T.L.; Darwish, H. Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J. Neuroinflam. 2012, 9, 244.
- El-Din, S.S.; Rashed, L.; Medhat, E.; Aboulhoda, B.E.; Badawy, A.D.; ShamsEldeen, A.M.; Abdelgwad, M. Active form of vitamin D analogue mitigates neurodegenerative changes in Alzheimer’s disease in rats by targeting Keap1/Nrf2 and MAPK-38p/ERK signaling pathways. Steroids 2020, 156, 108586.
- Ertilav, E.; Barcin, N.E.; Ozdem, S. Comparison of Serum Free and Bioavailable 25- Hydroxyvitamin D Levels in Alzheimer’s Disease and Healthy Control Patients. Lab. Med. 2020, 52, 219–225.
- Ali, A.; Shah, S.A.; Zaman, N.; Uddin, M.N.; Khan, W.; Ali, A.; Riaz, M.; Kamil, A. Vitamin D Exerts Neuroprotection via SIRT1/Nrf-2/ NF-kB Signaling Pathways against D-Galactose-induced Memory Impairment in Adult Mice. Neurochem. Int. 2020, 4, 104893.
- Fan, Y.G.; Pang, Z.Q.; Wu, T.Y.; Zhang, Y.H.; Xuan, W.Q.; Wang, Z.; Yu, X.; Li, Y.C.; Guo, C.; Wang, Z.Y. Vitamin D deficiency exacerbates Alzheimer-like pathologies by reducing antioxidant capacity. Free Radic. Biol. Med. 2020, 161, 139–149.
- Boccardi, V.; Baroni, M.; Mangialasche, F.; Mecocci, P. Vitamin E family: Role in the pathogenesis and treatment of Alzheimer’s disease. Alzheimers Dement. 2016, 2, 182–191.
- Yu, L.; Chen, Y.; Wang, W.; Xiao, Z.; Hong, Y. Multi-vitamin B supplementation reverses hypoxia-induced tau hyperphosphorylation and improves memory function in adult mice. J. Alzheimers Dis. 2016, 54, 297–306.
- Huang, J.K.; Jarjour, A.A.; Oumesmar, B.N.; Kerninon, C.; Williams, A.; Krezel, W.; Kagechika, H.; Bauer, J.; Zhao, C.; Baron-Van Evercooren, A. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 2011, 14, 45.
- Maden, M.; Holder, N. The involvement of retinoic acid in the development of the vertebrate central nervous system. Dev. Suppl. 1991, 113, 87–94.
- Chakrabarti, M.; McDonald, A.J.; Will Reed, J.; Moss, M.A.; Das, B.C.; Ray, S.K. Molecular signaling mechanisms of natural and synthetic retinoids for inhibition of pathogenesis in Alzheimer’s disease. J. Alzheimers Dis. 2016, 50, 335–352.
- Lane, M.A.; Bailey, S.J. Role of retinoid signalling in the adult brain. Prog. Neurobiol. 2005, 75, 275–293.
- Lee, H.-P.; Casadesus, G.; Zhu, X.; Lee, H.-G.; Perry, G.; Smith, M.A.; Gustaw-Rothenberg, K.; Lerner, A. All-trans retinoic acid as a novel therapeutic strategy for Alzheimer’s disease. Exp. Rev. Neurotherap. 2009, 9, 1615–1621.
- Niewiadomska-Cimicka, A.; Krzyżosiak, A.; Ye, T.; Podleśny-Drabiniok, A.; Dembélé, D.; Dollé, P.; Krężel, W. Genome-wide analysis of RARβ transcriptional targets in mouse striatum links retinoic acid signaling with Huntington’s disease and other neurodegenerative disorders. Mol. Neurobiol. 2017, 54, 3859–3878.
- Rataj-Baniowska, M.; Niewiadomska-Cimicka, A.; Paschaki, M.; Szyszka-Niagolov, M.; Carramolino, L.; Torres, M.; Dollé, P.; Krężel, W. Retinoic acid receptor β controls development of striatonigral projection neurons through FGF-dependent and Meis1- dependent mechanisms. J. Neurosci. 2015, 35, 14467–14475.
- Moutinho, M.; Codocedo, J.F.; Puntambekar, S.S.; Landreth, G.E. Nuclear Receptors as Therapeutic Targets for Neurodegenerative Diseases: Lost in Translation. Ann. Rev. Pharmacol. Toxicol. 2019, 59, 237–261.
- Li, J.Y.; Popovic, N.; Brundin, P. The use of the R6 transgenic mouse models of Huntington’s disease in attempts to develop novel therapeutic strategies. NeuroRx 2005, 2, 447–464.
- Liu, D.; Ke, Z.; Luo, J. Thiamine deficiency and neurodegeneration: The interplay among oxidative stress, endoplasmic reticulum stress, and autophagy. Mol. Neurobiol. 2017, 54, 5440–5448.
- Lonsdale, D. Thiamin and protein folding. Med. Hypotheses 2019, 129, 109252.
- Wang, X.; Xu, M.; Frank, J.A.; Ke, Z.J.; Luo, J. Thiamine deficiency induces endoplasmic reticulum stress and oxidative stress in human neurons derived from induced pluripotent stem cells. Toxicol. Appl. Pharmacol. 2017, 320, 26–31.
- Gruber-Bzura, B.M.; Krzysztoń-Russjan, J.; Bubko, I.; Syska, J.; Jaworska, M.; Zmysłowski, A.; Rosłon, M.; Drozd, J.; Drozd, E.; Majorczyk, E. Role of thiamine in Huntington’s disease pathogenesis: In vitro studies. Adv. Clin. Exp. Med. 2017, 26, 751–760.
- Sidhu, A.; Diwan, V.; Kaur, H.; Bhateja, D.; Singh, C.K.; Sharma, S.; Padi, S.S. Nicotinamide reverses behavioral impairments and provides neuroprotection in 3-nitropropionic acid induced animal model ofHuntington’s disease: Implication of oxidative stress- poly (ADP- ribose) polymerase pathway. Metab. Brain Dis. 2018, 33, 1911–1921.
- Blanchet, M.; Prince, F.; Chouinard, S.; Messier, J. Postural stability limits in manifest and premanifest Huntington’s disease under different sensory conditions. Neuroscience 2014, 279, 102–112.
- Montero-Odasso, M.; Pieruccini-Faria, F.; Bartha, R.; Black, S.E.; Finger, E.; Freedman, M.; Greenberg, B.; Grimes, D.A.; Hegele, R.A.; Hudson, C.; et al. Motor phenotype in neurodegenerative disorders: Gait and balance platform study design protocol for the ontario neurodegenerative research initiative (ONDRI). J. Alzheimers Dis. 2017, 59, 707–721.
- Wilczynski, J.; Pedrycz, A.; Mucha, D.; Ambrozy, T.; Mucha, D. Body posture, postural stability, and metabolic age in patients with Parkinson’s disease. BioMed Res. Int. 2017, 2017, 3975417.
- Aghajanian, P.; Hall, S.; Wongworawat, M.D.; Mohan, S. The roles and mechanisms of actions of vitamin C in bone: New developments. J. Bone Min. Res. 2015, 30, 1945–1955.
- Rebec, G.V.; Barton, S.J.; Marseilles, A.M.; Collins, K. Ascorbate treatment attenuates the Huntington behavioral phenotype in mice. Neuroreport 2003, 14, 1263–1265.
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells 2019, 8, 184.
- Rebec, G.V. Dysregulation of corticostriatal ascorbate release and glutamate uptake in transgenic models of Huntington’s disease. Antiox. Redox Sig. 2013, 19, 2115–2128.
- Dorner, J.L.; Miller, B.R.; Klein, E.L.; Murphy-Nakhnikian, A.; Andrews, R.L.; Barton, S.J.; Rebec, G.V. Corticostriatal dysfunction underlies diminished striatal ascorbate release in the R6/2 mouse model of Huntington’s disease. Brain Res. 2009, 1290, 111–120.
- Sahay, M.; Sahay, R. Rickets–vitamin D deficiency and dependency. Indian J. Endocrinol. Metab. 2012, 16, 164.
- Rejnmark, L. Effects of vitamin d on muscle function and performance: A review of evidence from randomized controlled trials. Ther. Adv. Chronic. Dis. 2011, 2, 25–37.
- Molnar, M.F.; Torok, R.; Szalardy, L.; Sumegi, E.; Vecsei, L.; Klivenyi, P. High-dose 1,25-dihydroxyvitamin D supplementation elongates the lifespan of Huntington’s disease transgenic mice. Acta Neurobiol. Exp. 2016, 76, 176–181.
- Peyser, C.E.; Folstein, M.; Chase, G.A.; Starkstein, S.; Brandt, J.; Cockrell, J.R.; Bylsma, F.; Coyle, J.T.; McHugh, P.R.; Folstein, S.E. Trial of d-!a-tocopherol in Huntington’s disease. Am. J. Psychiatry. 1995, 152, 1771–1775.
- Kašparová, S.; Sumbalová, Z.; Bystrický, P.; Kucharská, J.; Liptaj, T.; Mlynárik, V.; Gvozdjáková, A. Effect of coenzyme Q10 and vitamin E on brain energy metabolism in the animal model of Huntington’s disease. Neurochem. Int. 2006, 48, 93–99.
- Fragoso, Y.D.; Stoney, P.N.; McCaffery, P.J. The evidence for a beneficial role of vitamin A in multiple sclerosis. CNS Drugs 2014, 28, 291–299.
- Khosravi-Largani, M.; Pourvali-Talatappeh, P.; Rousta, A.M.; Karimi-Kivi, M.; Noroozi, E.; Mahjoob, A.; Asaadi, Y.; Shahmohammadi, A.; Sadeghi, S.; Shakeri, S.; et al. A review on potential roles of vitamins in incidence, progression, and improvement of multiple sclerosis. eNeurologicalsci 2018, 10, 37–44.
- Torkildsen, O.; Loken-Amsrud, K.I.; Wergeland, S.; Myhr, K.M.; Holmoy, T. Fat-soluble vitamins as disease modulators in multiple sclerosis. Acta Neurol. Scand. 2013, 127, 16–23.
- Warren, T.R. The increased prevalence of multiple sclerosis among people who were born and bred in areas where goitre is endemic. Med. Hypotheses 1984, 14, 111–114.
- Miller, E.D.; Dziedzic, A.; Saluk-Bijak, J.; Bijak, M. A review of various antioxidant compounds and their potential utility as complementary therapy in multiple sclerosis. Nutrients 2019, 11, 1528.
- Jafarirad, S.; Siassi, F.; Harirchian, M.-H.; Sahraian, M.-A.; Eshraghian, M.-R.; Shokri, F.; Amani, R.; Bitarafan, S.; Mozafari, S.; Saboor-Yaraghi, A. The effect of vitamin A supplementation on stimulated T-cell proliferation with myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. J. Neurosci. Rural Pract. 2012, 3, 294–298.
- Løken-Amsrud, K.I.; Myhr, K.-M.; Bakke, S.J.; Beiske, A.G.; Bjerve, K.S.; Bjørnarå, B.T.; Hovdal, H.; Lilleås, F.; Midgard, R.; Pedersen, T. Retinol levels are associated with magnetic resonance imaging outcomes in multiple sclerosis. Mult. Scler. J. 2013, 19, 451–457.
- Mizee, M.R.; Nijland, P.G.; van der Pol, S.M.; Drexhage, J.A.; van het Hof, B.; Mebius, R.; van der Valk, P.; van Horssen, J.; Reijerkerk, A.; de Vries, H.E. Astrocyte-derived retinoic acid: A novel regulator of blood–brain barrier function in multiple sclerosis. Acta Neuropathol. 2014, 128, 691–703.
- Salzer, J.; Hallmans, G.; Nyström, M.; Stenlund, H.; Wadell, G.; Sundström, P. Vitamin A and systemic inflammation as protective factors in multiple sclerosis. Mult. Scler. J. 2013, 19, 1046–1051.
- Runia, T.; Hop, W.; De Rijke, Y.; Hintzen, R. Vitamin A is not associated with exacerbations in multiple sclerosis. Mult. Scler. Rel. Dis. 2014, 3, 34–39.
- Evans, E.; Piccio, L.; Cross, A.H. Use of vitamins and dietary supplements by patients with multiple sclerosis: A review. JAMA Neurol. 2018, 75, 1013–1021.
- Besler, H.T.; Comoglu, S.; Okcu, Z. Serum levels of antioxidant vitamins and lipid peroxidation in multiple sclerosis. Nutr. Neurosci. 2002, 5, 215–220.
- Polachini, C.R.; Spanevello, R.M.; Zanini, D.; Baldissarelli, J.; Pereira, L.B.; Schetinger, M.R.; da Cruz, I.B.; Assmann, C.E.; Bagatini, M.D.; Morsch, V.M. Evaluation of delta-aminolevulinic dehydratase activity, oxidative stress biomarkers, and vitamin D levels in patients with multiple sclerosis. Neurotox. Res. 2016, 29, 230–242.
- Tavazzi, B.; Batocchi, A.P.; Amorini, A.M.; Nociti, V.; D’Urso, S.; Longo, S.; Gullotta, S.; Picardi, M.; Lazzarino, G. Serum metabolic profile in multiple sclerosis patients. Mult. Scler. Int. 2011, 2011, 167156.
- Babri, S.; Mehrvash, F.; Mohaddes, G.; Hatami, H.; Mirzaie, F. Effect of intrahippocampal administration of vitamin C and progesterone on learning in a model of multiple sclerosis in rats. Adv. Pharm. Bull. 2015, 5, 83.
- Hernández-Pedro, N.Y.; Espinosa-Ramirez, G.; De La Cruz, V.P.; Pineda, B.; Sotelo, J. Initial immunopathogenesis of multiple sclerosis: Innate immune response. Clin. Dev. Immunol. 2013, 2013, 413465.
- Zhang, S.M.; Hernan, M.A.; Olek, M.J.; Spiegelman, D.; Willett, W.C.; Ascherio, A. Intakes of carotenoids, vitamin C, and vitamin E and MS risk among two large cohorts of women. Neurology 2001, 57, 75–80.
- Najafi, M.R.; Shaygannajad, V.; Mirpourian, M.; Gholamrezaei, A. Vitamin B12 deficiency and multiple sclerosis; is there any association? Int. J. Prev. Med. 2012, 3, 286.
- Kruman, I.I.; Culmsee, C.; Chan, S.L.; Kruman, Y.; Guo, Z.; Penix, L.; Mattson, M.P. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J. Neurosci. 2000, 20, 6920–6926.
- Moghaddasi, M.; Mamarabadi, M.; Mohebi, N.; Razjouyan, H.; Aghaei, M. Homocysteine, vitamin B12 and folate levels in Iranian patients with multiple sclerosis: A case control study. Clin. Neurol. Neurosurg. 2013, 115, 1802–1805.
- Reynolds, E.; Bottiglieri, T.; Laundy, M.; Crellin, R.; Kirker, S. Vitamin B12 metabolism in multiple sclerosis. Arch. Neurol. 1992, 49, 649–652.
- Reynolds, E.; Linnell, J.; Faludy, J. Multiple sclerosis associated with vitamin B12 deficiency. Arch. Neurol. 1991, 48, 808–811.
- Weinstein, S.J.; Hartman, T.J.; Stolzenberg-Solomon, R.; Pietinen, P.; Barrett, M.J.; Taylor, P.R.; Virtamo, J.; Albanes, D. Null association between prostate cancer and serum folate, vitamin B6, vitamin B12, and homocysteine. Cancer Epid. Prev. Biomark. 2003, 12, 1271–1272.
- Lempriere, S. Vitamin B3 promotes remyelination. Nat. Rev. Neurol. 2020, 16, 184–185.
- Costantini, A.; Nappo, A.; Pala, M.I.; Zappone, A. High dose thiamine improves fatigue in multiple sclerosis. BMJ Case Rep. 2013, 2013, bcr2013009144.
- Bitarafan, S.; Saboor-Yaraghi, A.; Sahraian, M.A.; Nafissi, S.; Togha, M.; Moghadam, N.B.; Roostaei, T.; Siassi, F.; Eshraghian, M.R.; Ghanaati, H.; et al. Impact of vitamin A supplementation on disease progression in patients with multiple sclerosis. Arch. Iran Med. 2015, 18, 435–440.
- Loken-Amsrud, K.I.; Myhr, K.M.; Bakke, S.J.; Beiske, A.G.; Bjerve, K.S.; Bjornara, B.T.; Hovdal, H.; Lilleas, F.; Midgard, R.; Pedersen, T.; et al. Alpha-tocopherol and MRI outcomes in multiple sclerosis—Association and prediction. PLoS ONE 2013, 8, e54417.
- Gianforcaro, A.; Hamadeh, M.J. Dietary vitamin D3 supplementation at 10× the adequate intake improves functional capacity in the G93A transgenic mouse model of ALS, a pilot study. CNS Neurosci. Ther. 2012, 18, 547–557.
- Gianforcaro, A.; Solomon, J.A.; Hamadeh, M.J. Vitamin D(3) at 50× AI attenuates the decline in paw grip endurance, but not disease outcomes, in the G93A mouse model of ALS, and is toxic in females. PLoS ONE 2013, 8, e30243.
- Solomon, J.A.; Gianforcaro, A.; Hamadeh, M.J. Vitamin D3 deficiency differentially affects functional and disease outcomes in the G93A mouse model of amyotrophic lateral sclerosis. PLoS ONE 2011, 6, e29354.
- Libonati, L.; Onesti, E.; Gori, M.C.; Ceccanti, M.; Cambieri, C.; Fabbri, A.; Frasca, V.; Inghilleri, M. Vitamin D in amyotrophic lateral sclerosis. Funct. Neurol. 2017, 32, 35.
- Soto, C.; Satani, N. The intricate mechanisms of neurodegeneration in prion diseases. Trends Mol. Med. 2011, 17, 14–24.
- Kupfer, L.; Hinrichs, W.; Groschup, M. Prion protein misfolding. Curr. Mol. Med. 2009, 9, 826–835.
- Atkinson, C.J.; Zhang, K.; Munn, A.L.; Wiegmans, A.; Wei, M.Q. Prion protein scrapie and the normal cellular prion protein. Prion 2016, 10, 63–82.
- Terry, C.; Wadsworth, J.D. Recent Advances in Understanding Mammalian Prion Structure: A Mini Review. Front. Mol. Neurosci. 2019, 12, 169.
- Geschwind, M.D. Prion diseases. Continuum 2015, 21, 1612–1638.
- Benetti, F.; Biarnes, X.; Attanasio, F.; Giachin, G.; Rizzarelli, E.; Legname, G. Structural determinants in prion protein folding and stability. J. Mol. Biol. 2014, 426, 3796–3810.
- Prasad, K.N.; Bondy, S.C. Oxidative and inflammatory events in prion diseases: Can they be therapeutic targets? Curr. Aging Sci. 2019, 11, 216–225.
- Singh, N.; Singh, A.; Das, D.; Mohan, M.L. Redox control of prion and disease pathogenesis. Antioxid. Redox Signal. 2010, 12, 1271–1294.
- Briani, C.; Dalla Torre, C.; Citton, V.; Manara, R.; Pompanin, S.; Binotto, G.; Adami, F. Cobalamin deficiency: Clinical picture and radiological findings. Nutrients 2013, 5, 4521–4539.
- Calderon-Ospina, C.A.; Nava-Mesa, M.O. B Vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci. Ther. 2020, 26, 5–13.
- Rzepka, Z.; Respondek, M.; Pawlik, J.; Beberok, A.; Gryko, D.; Wrześniok, D. Cobalamin deficiency: Effect on homeostasis of cultured human astrocytes. Cells 2019, 8, 1505.
- Scalabrino, G. The multi-faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: Lessons learned from its deficiency. Prog. Neurobiol. 2009, 88, 203–220.
- Scalabrino, G.; Nicolini, G.; Buccellato, F.R.; Peracchi, M.; Tredici, G.; Manfridi, A.; Pravettoni, G. Epidermal growth factor as a local mediator of the neurotrophic action of vitamin B(12) (cobalamin) in the rat central nervous system. FASEB J. 1999, 13, 2083–2090.
- Scalabrino, G.; Veber, D. Cobalamin and normal prions: A new horizon for cobalamin neurotrophism. Biochimie 2013, 95, 1041–1046.
- Suenaga, M.; Hiramoto, Y.; Matsunaga, Y. Vitamin D2 interacts with Human PrPc (90–231) and breaks PrPc oligomerization in vitro. Prion 2013, 7, 312–318.
- Broadhead, G.K.; Grigg, J.R.; Chang, A.A.; McCluskey, P. Dietary modification and supplementation for the treatment of age-related macular degeneration. Nutr. Rev. 2015, 73, 448–462.
- Chew, E.Y.; Clemons, T.E.; Sangiovanni, J.P.; Danis, R.P.; Ferris, F.L., 3rd; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; Bressler, S.B.; et al. Secondary analyses of the effects of lute-in/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014, 132, 142–149.
- Tohari, A.M.; Zhou, X.; Shu, X. Protection against oxidative stress by vitamin D in cone cells. Cell Biochem. Funct. 2016, 34, 82–94.
- Kim, E.C.; Han, K.; Jee, D. Inverse relationship between high blood 25-hydroxyvitamin D and late stage of age-related macular degeneration in a repre-sentative Korean population. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4823–4831.
- Di Somma, C.; Scarano, E.; Barrea, L.; Zhukouskaya, V.V.; Savastano, S.; Mele, C.; Scacchi, M.; Aimaretti, G.; Colao, A.; Marzullo, P. Vitamin D and neurological diseases: An endocrine view. Int. J. Mol. Sci. 2017, 18, 2482.
- Fricker, R.A.; Green, E.L.; Jenkins, S.I.; Griffin, S.M. The influence of nicotinamide on health and disease in the central nervous system. Int. J. Trypt. Res. 2018, 11, 1178646918776658.
