Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
The advancement in therapy has provided a dramatic improvement in the rate of recovery among cancer patients. However, this improved survival is also associated with enhanced risks for cardiovascular manifestations, including hypertension, arrhythmias, and heart failure. The cardiotoxicity induced by chemotherapy is a life-threatening consequence that restricts the use of several chemotherapy drugs in clinical practice.
- anticancer drugs
- cardiotoxicity
- pharmacogenetics
- radiation therapy
- chemotherapeutic agent
1. Introduction
The advancement of medical science is associated with a paradigm shift in new anticancer therapies, causing a significant increase in long life expectancy in patients. Though a tremendous improvement has happened in cancer chemotherapy, the serious adverse effects associated with the therapy are still a major challenge [1][2][3]. Earlier studies reported a 0.5% prevalence rate of cancer in the general population with a mortality rate of 25%. The cytotoxic effects of the therapy affect all major organs and the clinical manifestations are associated with the development of co-morbidities [4]. Since cardiotoxicity is the most common adverse effect manifested by anticancer drug therapy, the increase in life expectancy owing to anticancer therapy may be negated by the enhanced death rate due to heart issues [5][6]. Cardiotoxicity can strike at any point during pharmacological treatment, with symptoms ranging from modest myocardial dysfunction to permanent heart failure and death [6].
The principle involved in chemotherapy is that it impairs the metabolic and mitotic processes of cancer cells and, in maximum cases, it also damages normal cells and tissues, which leads to various side effects ranging from mild to severe forms of gastrointestinal upset, the suppression of bone marrow, in addition to cardiovascular toxicities including myocardial dysfunction, heart failure, hypertension, and tachyarrhythmia [2][7].
Cancer therapies, including molecular target therapies, cytotoxic chemotherapy, and mediastinal irradiation, are seen to be linked with myocyte damage, ischemia, conduction and rhythm disturbances, left ventricular dysfunction, cardiac failure, and several other cardiovascular complications [3][8][9].
Various biochemical investigations into the different pathways of cardiovascular damage have been documented. Chemotherapeutic drugs cause cardiotoxicity by enhancing the formation of reactive oxygen and nitrogen species (ROS and RON), which impairs redox equilibrium. Although peroxisomes and other subcellular components are critical regulators of redox equilibrium, mitochondria remain the prime targets for anticancer-induced cardiotoxicity [10].
Anthracyclines (ANT) (doxorubicin), alkylating drugs (cyclophosphamide, cisplatin), and taxanes (paclitaxel, docetaxel) are the most common chemotherapeutic medications linked to serious cardiac events. More than half of the currently used anticancer therapies include anthracyclines, which cover breast cancer, sarcoma, gynecological cancer, and lymphoma [11].
2. Mode of Cardiotoxicity Induction
2.1. Cyclophosphamide-Induced Carditoxicity
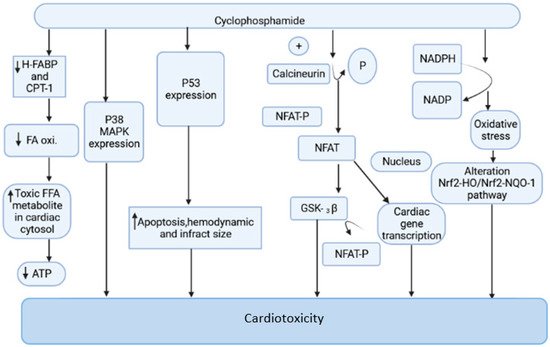
Cyclophosphamide is an anticancer drug with antitumor properties that is commonly used in humans for a range of neoplasms [12]. However, multiple reports have indicated that, in addition to having tumor-selective properties, cyclophosphamide has a slew of highly hazardous adverse manifestations (Figure 1), the most serious of which is cardiotoxicity [13].

Figure 1. The mechanism of cyclophosphamide-mediated cardiac toxicity.
Cyclophosphamide is activated by the cytochrome P-450 (CYP) enzyme in the liver, which transforms it into 4-hydroxycyclophosphamide. Aldo cyclophosphamide (AldoCY) and 4-hydroxycyclophosphamide are in equilibrium. AldoCY may be oxidized by aldehyde dehydrogenase 1 (ALDH1) to the inactive metabolite o-carboxymethylphosphoramide mustard (CEPM) or beta eliminated through a chemical mechanism that decomposes to produce cytotoxic phosphoramide mustard (PM) and the byproduct acrolein, depending on the cell type [14]. The antineoplastic activity of cyclophosphamide is due to the presence of phosphoramide mustard, which is the therapeutic metabolite in the active form present in cyclophosphamide, and it shows DNA alkylation activity, whereas acrolein, the other metabolite of cyclophosphamide, has the ability to interfere with the antioxidant system, further causing the generation of strongly active oxygen-free radicals, superoxide radicals, and hydrogen peroxide [15]. These free radicals are implicated in several enzyme inhibitions, lipid peroxidation, as well as membrane injury [16]. The generation of free radicals mediates oxidative stress associated with cyclophosphamide treatment witnessed by the following pathways.
2.1.1. Mitochondrial-Dependent ROS Production
Cyclophosphamide (CP) treatment is responsible for the generation of a tremendous amount of ROS, which damages the inner membrane of the mitochondria, resulting in a reduced capacity of the myocardial cell mitochondria to neutralize the toxic effect of ROS. The toxic effect of CP treatment is associated with a reduction in oxidative phosphorylation in mitochondrial cristae, which causes a decrease in ATP production and contributes to further damage in mitochondria [17][18][19]. This is one of the prime reasons for altered cardiac physiology and contractility. The increased expression of the pro-apoptotic molecule BAX in the mitochondrial membrane by CP treatment is responsible for the induction of apoptosis. The cleavage of caspases, inhibition of protein kinase, and the activation of phosphatase and increased intracellular PH are responsible for the stimulation of BAX proteins. The ratio of proapoptotic and antiapoptotic molecules Bax and Bcl2 makes the mitochondrial pathway susceptible to apoptosis. The disturbance in calcium homeostasis is also responsible for the initiation of apoptotic cell death [20][21]. The interaction of ROS and calcium is bidirectional. The calcium ions increase the production of mitochondrial ROS by stimulating respiratory chain activity. The released ROS acts on the endoplasmic reticulum to generate more calcium and ROS. This leads to the opening of the mitochondrial permeability transition pore, resulting in the release of pro-apoptotic factors [22][23].
2.1.2. Oxidative Stress Produced by NADPH
CP-intoxicated cardiomyocytes are responsible for increased synthesis of NADPH oxidase, NADH dehydrogenase, and NADPH oxidase. The activation of these enzymatic systems is associated with the generation of free radical-mediated oxidative stress [24][25]. The myocardial cell when exposed to CP treatment increases NADPH oxidase and other mediators of ROS. The oxidative stress generated by the NADPH-mediated pathway is responsible for the alteration of the Nrf2-HO/Nrf2-NQO-1 pathway, which causes damage in the myocardial cell [16][25][26].
2.2. Doxorubicin-Induced Cardiotoxicity
Doxorubicin (DOX) is an anthracycline antibiotic that was first derived from a bacterium called Streptomyces peucetius in the early 1960s and was first used as a cytotoxic drug in 1969 [27][28]. It is a highly successful chemotherapeutic medication for solid tumors, soft-tissue sarcoma, breast cancer, Hodgkin’s disease, Kaposi’s sarcoma, acute lymphoblastic leukemia, pediatric leukemia, lung cancer, lymphomas, and various metastatic malignancies [2][29][30]. The drug’s utility is limited owing to its large number of side effects, such as the suppression of the hematopoietic system, gastrointestinal disturbances, and baldness, with cardiac damage being the most dangerous [31][32][33].
The advanced conditions of cancer patients treated with repeated doses of doxorubicin for more than a month developed severe symptoms of myocardial toxicity with a prevalent rate of more than 30%. The wide spread of symptoms ranged from ventricular failure, a decrease in QRS segment, cardiac dilatation, tachycardia (150 beats/min), and hypotension (blood pressure 70/50 mmHg) [34]. The patients were unresponsive to inotropic drugs and mechanical circulatory assist devices. Several biomarker levels such as creatine phosphokinase, serum glutamic-oxaloacetic transaminase, and lactate dehydrogenase were also elevated. The histopathological examination reported decreased myofibrils, altered structure of the sarcoplasmic reticulum, vacuolization of the cytoplasm, mitochondrial inflammation, and elevated numbers of lysosomes [35][36]. Different experimental animals such as rats, mice, and rabbits treated with doxorubicin also reported similar symptoms of myocardial toxicities. The experimental animals in several studies in a reproducible manner showed the symptoms of cardiomyopathy and heart failure with the exposure of doxorubicin [37][38].
Multiple processes are involved in doxorubicin-induced cardiotoxicity, which has been linked to increased myocardial damage due to oxidative free radicals, as well as lower levels of sulfhydryl groups and antioxidants. In addition to myofibrillar deterioration and intracellular calcium dysregulation, doxorubicin-induced cardiac toxicity is known to cause myofibrillar deterioration [39][40]. Mitochondrial biogenesis is also hypothesized to be actively involved in doxorubicin-mediated cardiac damage due to its potential to stimulate the cell death pathway while blocking topoisomerase 2β [41][42]. Alterations in gene expression of the cardiac system, such as the expression of muscle-specific genes (cardiac actin, myosin light chain, and muscle creatine kinase), are shown to decrease in response to doxorubicin exposure in acute doxorubicin cardiotoxicity [43][44]. Endothelial dysfunction, an activated ubiquitin protease system, autophagy, and cell death collectively participate in doxorubicin-induced cardiotoxicity, as do NO release, impaired adenosine triphosphate (ATP) levels, iron regulatory protein (IRP) production, and augmented inflammatory mediator release [45][46].
2.2.1. Mechanism of Doxorubicin-Induced Cardiotoxicity
Oxidative Stress
The generation of a tremendous amount of oxidative stress associated with DOX is the main culprit for the degeneration of myocardial cells. The imbalance between reactive oxygen species, reactive nitrogen species, and the intrinsic antioxidant systems is responsible for the development of oxidative stress. The following are some of the important cellular mechanisms that are responsible for the development of this oxidative stress [47][48].
- A.
-
Altered mitochondrial functions
The mitochondria fulfill the huge oxygen demand of cardiomyocytes. DOX treatment is responsible for structural changes in the mitochondria responsible for the depletion of energy production in the form of ATP [49][50].
The inner membrane of mitochondria contains an important component—cardiolipin. The interaction between cardiolipin and DOX is one of the major events responsible for cardiotoxicity associated with DOX. DOX and cardiolipin bind irreversibly due to the cationic charge of DOX and the anionic charge of cardiolipin, resulting in the accumulation of DOX in mitochondria. Cardiolipin has a major role in electron transport, but due to the formation of a complex with DOX, the activation of several enzymes is inhibited, resulting in an altered electron transport chain. Apart from this, DOX-mediated oxidative phosphorylation also plays an important role in the events of myocardial toxicity [51][52].
- B.
-
Fe–Dox complex
The hydroxy and ketone groups of DOX interact with Ferric ion (Fe3+) and form a complex. This complex interacts with the cell membrane and causes lipid peroxidation and generates free radicals. DOX is responsible for the accumulation of iron in mitochondria and initiates the event of apoptosis in the myocardial cell. DOX treatment is responsible for the inactivation of iron regulatory protein IRP1, and IRP2 and iron are transported into mitochondria by transport protein Mitoferrin-2. DOX causes the alteration of the post-translational modification of IRP1 and the recognition of iron-responsive elements is lost and results in altered iron homeostasis [53][54].
- C.
-
Role of NADPH in the generation of ROS
The catalytic activities of the enzymes nicotinamide adenosine dinucleotide phosphate (NADPH) and mitochondrial NADH dehydrogenase are responsible for the generation of free radicals. Angiotensin II is responsible for the elevation of NADPH oxidase and having a pivotal role in the generation of free radicals. DOX treatment is associated with the incremental genesis of nitric oxide synthase; nitric oxide reductase and P450 reductase enzymes contribute to the development of oxidative stress in the myocardial cell due to the formation of reactive oxygen species [55][56].
- D.
-
Generation of reactive oxygen species by nitric oxide
DOX treatment is responsible for the increased synthesis of nitric oxide mediated by the enzymes neuronal NO synthase, inducible NO synthase, and endothelial NO synthase. Nitric oxide is found in increased levels in damaged myocardial tissues. Nitric oxide by lipid peroxidation from peroxynitrite is responsible for the development of oxidative stress in mitochondria, resulting in necrosis and apoptosis [57][58].
- E.
-
Generation of oxidative stress by nrf2
The depletion of Nrf2 protein in DOX treatment is responsible for associated myocardial toxicities. The expression of Nrf2 is responsible for the induction of autophagy and maintains homeostasis between autophagy and oxidative stress [59].
Apoptosis
Through both the intrinsic and extrinsic pathways, DOX stimulates the apoptosis of the cardiac muscle cells. DOX toxicity results in an imbalance in various factors including an increase in oxidative stress, which activates HSF-1 (heat shock factor 1), thereby inducing HSP-25 (heat shock protein), and subsequently, p53 is equalized, leading to the production of proapoptotic factors such as Fas, FasL, and c-Myc, which is responsible for cardiac muscle cell death [60][61]. Another factor responsible for the cardiotoxicity associated with DOX is DOX-induced cardiotoxicity leading to the depletion of transcriptional factor GATA-4, which is responsible for regulating the apoptotic pathway via activating the anti-apoptotic gene Bcl-XL. In addition, DOX-induced cardiotoxicity is also found to increase active glycogen synthase kinase 3β (GSK3β), which is a negative regulator of GATA-4 in the nucleus [62]. Similarly, a role of TLR-2 (Toll-like receptor-2)-mediated cytokine production, cardiac dysfunction, and apoptosis by the activation of the pro-inflammatory nuclear factor-κB (NF-κB) pathway has been observed to be involved in DOX-induced cardiotoxicity [30]. Some studies showed that DOX-treated murine hearts inhibit Protein Kinase B (PKB/AKT), which is involved in the regulation of cell survival, proliferation, and metabolism. AKT is also found to play a critical role in decreasing oxidative stress via deactivating (GSK3β), which thereby decreases FYN nuclear translocation-mediated NF-E2-related factor 2 (Nrf2) nuclear export and degradation [63][64].
2.3. Trastuzumab-Induced Cardiotoxicity
This is a monoclonal antibody that is employed for the treatment of breast cancer patients who concurrently have an elevated human epidermal growth factor receptor 2 (HER2, also known as ErbB-21) level [65]. While therapeutic therapy with trastuzumab is reported to reduce morbidity and mortality in breast cancer patients, there are serious cardiac adverse effects that need to be checked [66][67]. HER2 is an important target for breast cancer and, similarly, a prime target for trastuzumab [68]. However, some mutant mouse models have documented that there are also some important roles regulated by ErbB-2 genes, such as in postnatal cardiomyocyte function and development [69]. Similarly, evidence suggests that ErbB-2 genes are involved in the normal functioning of the myocardium, which includes the involvement of a number of key pathways (such as phosphoinositide 3-kinase, mitogen-activated protein kinase, and focal adhesion kinase) that are required for cardiomyocyte maintenance, and the inhibition of apoptosis. Therefore, the blockade of ErbB signaling by trastuzumab could have been a major reason for its ability to induce cardiotoxicity [70][71].
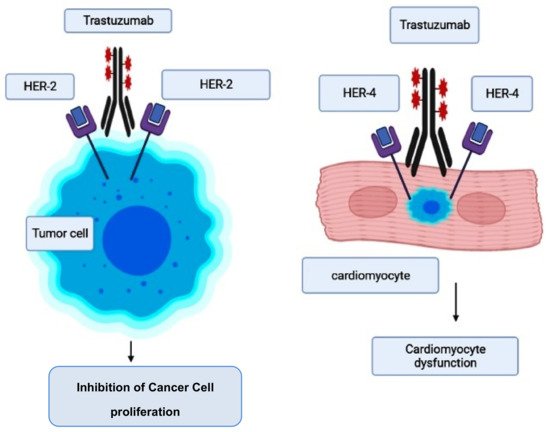
However, the risk of developing trastuzumab cardiotoxicity has been seen to increase in patients who receive concurrent anthracycline therapy [67][72][73]. When trastuzumab suppresses ErbB2 signaling, it was reported that it further accelerates the anthracycline’s ability to induce sarcomeric protein breakdown, increasing the chances of cardiac toxicity and heart failure [74]. The pathogenesis and molecular mechanism involved in trastuzumab-induced cardiotoxicity are given in Figure 2 [75].

Figure 2. Mode of action of trastuzumab and its cardiotoxicity induction.
2.4. Fluorouracil-Induced Cardiotoxicity
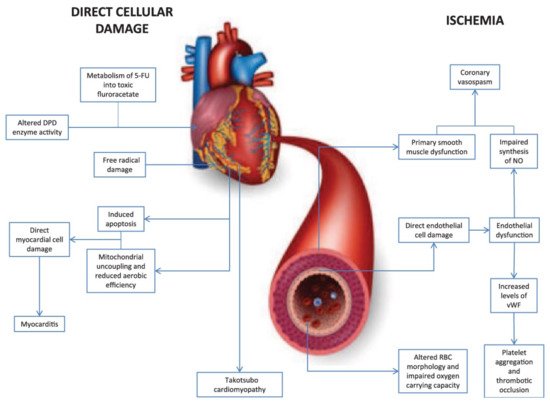
5-fluorouracil (5-FU) is a pyrimidine analogue chemotherapy medication employed in the treatment of a number of cancer types, including colorectal, breast, gastric, pancreatic, prostate, and bladder cancers, among others [76][77]. Myelosuppression, diarrhea, stomatitis, nausea, vomiting, and baldness are common side effects of the medication. Furthermore, 5-FU has been linked to cardiotoxicity, which includes myocardial infarction, cardiac arrhythmias, altered blood pressure, left ventricular failure, cardiac arrest, and sudden death [76]. The potential mechanisms involved in 5-FU-induced cardiotoxicity are given in Figure 3 [78]. Angina is noticeable mostly during infusions, and occasionally it is delayed until a few hours after 5-FU application. The incidence of angina associated with the use of 5-FU ranges from 1.2 to 18 percent [79][80][81][82]. Cardiotoxicity that is severe or life-threatening or ventricular arrhythmias, on the other hand, are far less common, with an incidence of about 0.55 percent [83]. When compared to anthracyclines, cardiac adverse effects of 5-FU are rare, with an incidence of 1.2–7.6%, and life-threatening cardiotoxicity of 5-FU has been documented in less than 1% of cases [84]. Though the exact processes of 5-FU-mediated cardiac toxicity are yet unknown, spasm of the coronary artery is suggested as a hypothesis [55]. Ultrasound and angiography have been used in several investigations to show that 5-FU infusion causes both coronary and brachial artery vasospasm. Patients with coronary vasospasm may have ECG findings suggestive of coronary occlusion, such as ST-segment elevation and biochemical changes in myocardial injury with increased troponin. As a result, it is recommended that when 5-FU is given to cancer patients, a high index of suspicion for probable cardiac toxicity be maintained [85].

Figure 3. Diagrammatic representation of two major mechanisms of 5-fluorouracil-induced cardiotoxicity.
2.5. Cisplatin-Induced Cardiotoxicity
Cisplatin, commonly known as cis-di ammine dichloroplatinum (CDDP), is a highly effective chemotherapy drug [86]. In diseases such as ovarian and cervical cancer and testicular cancer, it is used alone or in combination regimens [87]. However, due to adverse effects, including toxicities to the kidney, liver, and gastrointestinal disturbances, cisplatin’s clinical use is restricted. Despite these side effects, many survivors of cisplatin treatment may develop acute or chronic cardiovascular problems, which can negatively impact their quality of life [88]. CDDP-induced cardiotoxicity has been linked to ventricular and supraventricular arrhythmias, occasional sinus bradycardia, alterations in electrocardiography, occasional total atrioventricular block, and congestive heart failure. With cisplatin-based chemotherapy, oxidative stress is considered as a major reason for cardiac toxicity [89]. It has also been shown that cisplatin-based chemotherapy causes a decrease in the concentration of different antioxidants in patients. It may also cause ROS generation by accumulating in mitochondria [90]. However, it has been documented that all of these factors collectively lead to congestive heart failure and sudden cardiac death. Table 1 summarizes the adverse cardiotoxic consequences of chemotherapy [91].
Table 1. Different types of cardiotoxic effects of chemotherapeutic agents.
| Drugs Causing Ischemia or Thromboembolism | Cisplatin, Thalidomide, Fluorouracil, Capecitabine, Paclitaxel, Docetaxel, Trastuzumab, Anthracyclines/Anthraquinones, Cyclophosphamide |
|---|---|
| Drugs that Cause Hypertension | bevacizumab, cisplatin, sunitinib, sorafenib |
| Tamponade and Endomyocardial Fibrosis | busulfan |
| Autonomic Neuropathy | vincristine |
| Bradyarrhythmias | paclitaxel |
| Myocarditis with Hemorrhage (rare) | cyclophosphamide (high-dose therapy) |
| Pulmonary Fibrosis | bleomycin, methotrexate, busulfan, cyclophosphamide |
| Raynaud’s Phenomenon | vinblastine, bleomycin |
| Torsades de Pointes or QT Prolongation | arsenic trioxide |
2.6. Immunotherapy-Induced Cardiotoxicity
With cancer progression, initially, the body’s immune system prevents tumor outgrowth. However, cancer cells can escape the various pathways that provide immunologic antitumor responses, and such pathways include immune system inhibitory pathways such as cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), programmed cell death ligand 1 (PD-L1), and programmed cell death 1 (PD-1) (natural checkpoints that dampen the antitumor responses of T cells). Therefore, various studies were carried out to determine if by stimulating an antitumor immune response in cancer patients, cancer could be fought better. Since then, immunotherapy came into existence, and this is divided into passive immunotherapies such as monoclonal antibodies, checkpoint inhibitors, cytokine therapy, bispecific T cell engager and antitumor vaccines or adoptive T cell transfer, which dramatically improved outcomes for wide varieties of cancer, among which adoptive T cell therapy and immune checkpoint inhibitors are most widely used and are used as longer duration therapy. However, having the benefit of activated immune response came with a price, as both the therapies have been reported to cause various cardiovascular complications such as hypotension, arrhythmia, left ventricular dysfunction, myocarditis, with clinical presentations ranging from asymptomatic cardiac biomarker elevation to heart failure, and cardiogenic shock [92][93][94][95][96].
2.6.1. Immune Checkpoint Inhibitors and Cardiac Complications
Immune checkpoint inhibitors (ICIs) show their action by amplifying T cell-mediated immune response against cancer cells, by blocking the intrinsic down-regulators of immunity, such as programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte antigen 4 (CTLA-4). These immune checkpoint inhibitors can induce tumor responses in different types of tumors including non-small cell lung cancer, renal cancer, melanoma, and Hodgkin’s disease. Although ICIs have brought improvement in the treatment of many aggressive malignancies, several studies have reported that PD-1 deletion and CTLA-4 inhibition can cause serious cardiac complications. When an experiment was carried out in a mouse model, it was reported that with the loss of the PD-1 or CTLA-4 receptor, significant infiltration of CD4+ and CD8+ T cells took place, resulting in the development of a dilated cardiomyopathy. However, the exact mechanism underlying cardiac toxicity associated with checkpoint inhibitors has not been studied comprehensively, and immune-mediated myocarditis could also be the exaggerated adaptive immune response against shared epitopes in the myocardium and tumor cells [97][98][99][100][101].
2.6.2. CDK4/6 Inhibitors
Cyclins have a major role to play in regulating the cell cycle. They show their activity by interacting with their partner serine/threonine CDKs (cyclin-dependent kinases). There are different types of CDKs available. CDKs 1–6 have a major role in coordinating cell cycle progression, whereas CDKs 7, 8, and 9 show downstream effects as transcriptional regulators. Among all the CDKs, the major target in cancer therapy is CDK 4/6, since it is required for the initiation and progression of various malignancies and is usually hyperactive in cancers [102][103]. CDKs generally help in regulating the cellular transition from G1 phase to S1 phase in the cell cycle, and the inhibitors (CDK 4/6 inhibitors) show their action by blocking the proliferation of cancer cells by effectively inducing G1 cell cycle arrest. However, CDK inhibitors have been seen to cause cardiac complications. One major concern about the use of CDK inhibitors is that they have been seen to potentially increase the QTc interval, which is most commonly seen with ribociclib. Some studies showed that ribociclib is capable of down-regulating the expression of KCNH2 (which encodes for the potassium channel Herg) and up-regulating the expression of SCN5A and SNTA1 (which encode for the sodium channels syntrophin-α1 and Nav1.5), which are the genes associated with long QT syndrome. There is also evidence that shows the increasing risk of thromboembolic events with the use of CDK inhibitors. However, there are limited data available regarding the cardiac safety of CDK 4/6 inhibitors. Therefore, it requires detailed investigation to understand the exact mechanism [104][105].
This entry is adapted from the peer-reviewed paper 10.3390/ph14100970
References
- Chaulin, A.M.; Abashina, O.E.; Duplyakov, D.V. Pathophysiological mechanisms of cardiotoxicity in chemotherapeutic agents. Russ. Open Med. J. 2020, 9, 1–9.
- Moudgil, R.; Yeh, E.T. Mechanisms of cardiotoxicity of cancer chemotherapeutic agents: Cardiomyopathy and beyond. Can. J. Cardiol. 2016, 32, 863–870.
- Dong, J.; Chen, H. Cardiotoxicity of anticancer therapeutics. Front. Cardiovasc. Med. 2018, 7, 5–9.
- Grieve, D.J.; Davidson, S.M. New insights into cardiotoxicity caused by chemotherapeutic agents: Editorial. Br. J. Pharmacol. 2017, 174, 3675–3676.
- Jain, D.; Aronow, W. Cardiotoxicity of cancer chemotherapy in clinical practice. Hosp. Pract. 2019, 47, 6–15.
- Rhea, I.B.; Oliveira, G.H. Cardiotoxicity of novel targeted chemotherapeutic agents. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 1.
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26.
- Bloom, M.W.; Hamo, C.E.; Cardinale, D.; Ky, B.; Nohria, A.; Baer, L.; Skopicki, H.; Lenihan, D.J.; Gheorghiade, M.; Lyon, A.R.; et al. Cancer therapy–related cardiac dysfunction and heart failure. Circ. Heart Fail. 2016, 9, 1–10.
- Perez, I.E.; Taveras Alam, S.; Hernandez, G.A.; Sancassani, R. Cancer therapy-related cardiac dysfunction: An overview for the clinician. Clin. Med. Insights Cardiol. 2019, 13.
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.E.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S.; et al. Cardiovascular toxicity related to cancer treatment: A pragmatic approach to the American and European cardio-oncology guidelines. J. Am. Heart Assoc. 2020, 9, e018403.
- Bansal, N.; Adams, M.J.; Ganatra, S.; Colan, S.D.; Aggarwal, S.; Steiner, R.; Amdani, S.; Lipshultz, E.R.; Lipshultz, S.E. Strategies to prevent anthracycline-induced cardiotoxicity in cancer survivors. Cardio-Oncology 2019, 5, 1–22.
- Bhatt, L.; Sebastian, B.; Joshi, V. Mangiferin protects rat myocardial tissue against cyclophosphamide-induced cardiotoxicity. J. Ayurveda Integr. Med. 2017, 8, 62–67.
- Ayza, M.A.; Zewdie, K.A.; Tesfaye, B.A.; Wondafrash, D.Z.; Berhe, A.H. The role of antioxidants in ameliorating cyclophosphamide-induced cardiotoxicity. Oxid. Med. Cell Longev. 2020, 2020, 1–14.
- Iqubal, A.; Iqubal, M.K.; Sharma, S.; Ansari, M.A.; Najmi, A.K.; Ali, S.M.; Ali, J.; Haque, S.E. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: Old drug with a new vision. Life Sci. 2019, 218, 112–131.
- Jeelani, R.; Khan, S.N.; Shaeib, F.; Kohan-Ghadr, H.-R.; Aldhaheri, S.R.; Najafi, T.; Thakur, M.; Morris, R.; Abu-Soud, H.M. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality. Free Radic. Biol. Med. 2017, 110, 11–18.
- Ayza, M.A.; Balasubramanian, R.; Berhe, A.H. Cardioprotective effect of Croton macrostachyus stem bark extract and solvent fractions on cyclophosphamide-induced cardiotoxicity in rats. Evid. Based Complement. Altern. Med. 2020, 2020, 8467406.
- Kohlhaas, M.; Nickel, A.G.; Maack, C. Mitochondrial energetics and calcium coupling in the heart. J. Physiol. 2017, 595, 3753–3763.
- McLaughlin, D.; Zhao, Y.; O’Neill, K.M.; Edgar, K.S.; Dunne, P.D.; Kearney, A.M.; Grieve, D.J.; McDermott, B.J. Signalling mechanisms underlying doxorubicin and Nox2 NADPH oxidase-induced cardiomyopathy: Involvement of mitofusin-2. Br. J. Pharmacol. 2017, 174, 3677–3695.
- Varghese, M.V.; Abhilash, M.; Paul, M.S.; Alex, M.; Nair, R.H. Omega-3 fatty acid protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Cardiovasc. Toxicol. 2017, 17, 109–119.
- Hamm, N.C.; Stammers, A.N.; Susser, S.E.; Hlynsky, M.W.; Kimber, D.E.; Kehler, D.S.; Duhamel, T.A. Regulation of cardiac sarco (endo) plasmic reticulum calcium-ATPases (SERCA2a) in response to exercise. In Regulation of Ca2+-ATPases, V-ATPases and F-ATPases; Springer: Cham, Switzerland, 2016; pp. 187–206.
- Fabris, S.; MacLean, D.A. Doxorubicin chemotherapy affects intracellular and interstitial nitric oxide concentrations in skeletal muscle. Cell Biol. Toxicol. 2016, 32, 121–131.
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.R.; Harrison, D.G.; Bhatnagar, A. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: A scientific statement from the American Heart Association. Circ. Res. 2016, 119, e39–e75.
- Kurauchi, K.; Nishikawa, T.; Miyahara, E.; Okamoto, Y.; Kawano, Y. Role of metabolites of cyclophosphamide in cardiotoxicity. BMC Res. Notes 2017, 10, 1–10.
- Komolafe, O.A.; Arayombo, B.E.; Abiodun, A.A.; Saka, O.S.; Abijo, A.Z.; Ojo, S.K.; Fakunle, O.O. Immunohistochemical and histological evaluations of cyclophosphamide-induced acute cardiotoxicity in wistar rats: The role of turmeric extract (curcuma). Morphologie 2020, 104, 133–142.
- Elrashidy, R.A.; Hasan, R.A. Cilostazol preconditioning alleviates cyclophosphamide-induced cardiotoxicity in male rats: Mechanistic insights into SIRT1 signaling pathway. Life Sci. 2021, 266, 118822.
- El-Agamy, D.S.; Elkablawy, M.A.; Abo-Haded, H.M. Modulation of cyclophosphamide-induced cardiotoxicity by methyl palmitate. Cancer Chemother. Pharmacol. 2017, 79, 399–409.
- Gazia, M.A.; El-Magd, M.A. Ameliorative effect of cardamom aqueous extract on doxorubicin-induced cardiotoxicity in rats. Cells Tissues Organs 2018, 206, 62–72.
- Songbo, M.; Lang, H.; Xinyong, C.; Bin, X.; Ping, Z.; Liang, S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48.
- Koleini, N.; Kardami, E. Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity. Oncotarget 2017, 8, 46663.
- Wenningmann, N.; Knapp, M.; Ande, A.; Vaidya, T.R.; Ait-Oudhia, S. Insights into doxorubicin-induced cardiotoxicity: Molecular mechanisms, preventive strategies, and early monitoring. Mol. Pharmacol. 2019, 96, 219–232.
- Renu, K.; Abilash, V.G.; Pichiach, B.T.P.; Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy—An update. Eur. J. Pharmacol. 2018, 818, 241–253.
- Podyacheva, E.Y.; Kushnareva, E.A.; Karpov, A.A.; Toropova, Y.G. Analysis of models of doxorubicin-induced cardiomyopathy in rats and mice. A modern view from the perspective of the pathophysiologist and the clinician. Front. Pharmacol. 2021, 12, 1398.
- Singal, P.; Li, T.; Kumar, D.; Danelisen, I.; Iliskovic, N. Adriamycin-induced heart failure: Mechanisms and modulation. Mol. Cell. Biochem. 2000, 207, 77–86.
- Mobaraki, M.; Faraji, A.; Zare, M.; Dolati, P.; Ataei, M.; Manshadi, H.D. Molecular mechanisms of cardiotoxicity: A review on major side-effect of doxorubicin. Indian J. Pharm. Sci. 2017, 79, 335–344.
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708.
- Shabalala, S.; Muller, C.J.; Louw, J.; Johnson, R. Polyphenols, autophagy and doxorubicin-induced cardiotoxicity. Life Sci. 2017, 180, 160–170.
- Koleini, N.; Nickel, B.E.; Edel, A.L.; Fandrich, R.R.; Ravandi, A.; Kardami, E. Oxidized phospholipids in Doxorubicin-induced cardiotoxicity. Chem. Biol. Interact. 2019, 303, 35–39.
- Abdullah, C.S.; Alam, S.; Aishwarya, R.; Miriyala, S.; Bhuiyan, M.A.; Panchatcharam, M.; Pattillo, C.B.; Orr, A.W.; Sadoshima, J.; Hill, J.A.; et al. Doxorubicin-induced cardiomyopathy associated with inhibition of autophagic degradation process and defects in mitochondrial respiration. Sci. Rep. 2019, 9, 1–20.
- Al Qahtani, A.A.; Osman, A.M.; Damanhouri, Z.A.; Al-Kreathy, H.M.; Al-Malky, H.S.; Ramadan, W.S.; Alharthi, S.E.; Kamel, F.O. Cardioprotective effect of marine Astaxanthin on doxorubicin-induced cardiotoxicity in normal rats. J. Pharm. Res. Int. 2019, 11, 1–11.
- Zhao, L.; Qi, Y.; Xu, L.; Tao, X.; Han, X.; Yin, L.; Peng, J. MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biol. 2018, 15, 284–296.
- Corremans, R.; Adão, R.; De Keulenaer, G.W.; Leite-Moreira, A.F.; Brás-Silva, C. Update on pathophysiology and preventive strategies of anthracycline-induced cardiotoxicity. Clin. Exp. Pharmacol. Physiol. 2019, 46, 204–215.
- Wu, Y.Z.; Zhang, L.; Wu, Z.X.; Shan, T.T.; Xiong, C. Berberine ameliorates doxorubicin-induced cardiotoxicity via a SIRT1/p66Shc-mediated pathway. Oxid. Med. Cell. Longev. 2019, 2019, 339.
- Zhang, Y.Y.; Yi, M.; Huang, Y.P. Oxymatrine ameliorates doxorubicin-induced cardiotoxicity in rats. Cell. Physiol. Biochem. 2017, 43, 626–635.
- Alkuraishy, H.M.; Al-Gareeb, A.I.; Al-hussaniy, H.A. Doxorubicin-induced cardiotoxicity: Molecular mechanism and protection by conventional drugs and natural products. Int. J. Clin. Oncol. Cancer Res. 2017, 2, 31–44.
- Kalyanaraman, B. Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: Have we been barking up the wrong tree? Redox Biol. 2020, 29, 101394.
- Hu, C.; Zhang, X.; Song, P.; Yuan, Y.P.; Kong, C.Y.; Wu, H.M.; Xu, S.C.; Ma, Z.G.; Tang, Q.Z. Meteorin-like protein attenuates doxorubicin-induced cardiotoxicity via activating cAMP/PKA/SIRT1 pathway. Redox Biol. 2020, 37, 101747.
- Zhang, X.; Hu, C.; Kong, C.Y.; Song, P.; Wu, H.M.; Xu, S.C.; Yuan, Y.P.; Deng, W.; Ma, Z.G.; Tang, Q.Z. FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ. 2020, 27, 540–555.
- Chang, V.Y.; Wang, J.J. Pharmacogenetics of chemotherapy-induced cardiotoxicity. Curr. Oncol. Rep. 2018, 20, 1–8.
- Zheng, X.; Zhong, T.; Ma, Y.; Wan, X.; Qin, A.; Yao, B.; Zou, H.; Song, Y.; Yin, D. Bnip3 mediates doxorubicin-induced cardiomyocyte pyroptosis via caspase-3/GSDME. Life Sci. 2020, 242, 2–9.
- Zilinyi, R.; Czompa, A.; Czegledi, A.; Gajtko, A.; Pituk, D.; Lekli, I.; Tosaki, A. The cardioprotective effect of metformin in doxorubicin-induced cardiotoxicity: The role of autophagy. Molecules 2018, 23, 1184.
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 1–5.
- Ruggeri, C.; Gioffré, S.; Achilli, F.; Colombo, G.I.; D’Alessandra, Y. Role of microRNAs in doxorubicin-induced cardiotoxicity: An overview of preclinical models and cancer patients. Heart Fail. Rev. 2018, 23, 109–122.
- Timm, K.N.; Perera, C.; Ball, V.; Henry, J.A.; Miller, J.J.; Kerr, M.; West, J.A.; Sharma, E.; Broxholme, J.; Logan, A.; et al. Early detection of doxorubicin-induced cardiotoxicity in rats by its cardiac metabolic signature assessed with hyperpolarized MRI. Commun. Biol. 2020, 3, 1–10.
- Tadokoro, T.; Ikeda, M.; Ide, T.; Deguchi, H.; Ikeda, S.; Okabe, K.; Ishikita, A.; Matsushima, S.; Koumura, T.; Yamada, K.I.; et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight 2020, 5, e132747.
- Osataphan, N.; Phrommintikul, A.; Chattipakorn, S.C.; Chattipakorn, N. Effects of doxorubicin-induced cardiotoxicity on cardiac mitochondrial dynamics and mitochondrial function: Insights for future interventions. J. Cell. Mol. Med. 2020, 24, 6534–6557.
- Mancilla, T.R.; Iskra, B.; Aune, G.J. Doxorubicin-induced cardiomyopathy in children. Compr. Physiol. 2019, 9, 905.
- Gallo, S.; Spilinga, M.; Albano, R.; Ferrauto, G.; Di Gregorio, E.; Casanova, E.; Balmativola, D.; Bonzano, A.; Boccaccio, C.; Sapino, A.; et al. Activation of the MET receptor attenuates doxorubicin-induced cardiotoxicity in vivo and in vitro. Br. J. Pharmacol. 2020, 177, 3107–3122.
- Lee, Y.; Kwon, I.; Jang, Y.; Cosio-Lima, L.; Barrington, P. Endurance exercise attenuates doxorubicin-induced cardiotoxicity. Med. Sci. Sports Exerc. 2020, 52, 25–36.
- Xu, Z.M.; Li, C.B.; Liu, Q.L.; Li, P.; Yang, H. Ginsenoside Rg1 prevents doxorubicin-induced cardiotoxicity through the inhibition of autophagy and endoplasmic reticulum stress in mice. Int. J. Mol. Sci. 2018, 19, 3658.
- Yarmohammadi, F.; Rezaee, R.; Karimi, G. Natural compounds against doxorubicin-induced cardiotoxicity: A review on the involvement of Nrf2/ARE signaling pathway. Phytother. Res. 2021, 35, 1163–1175.
- Qi, W.; Boliang, W.; Xiaoxi, T.; Guoqiang, F.; Jianbo, X.; Gang, W. Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomed. Pharmacother. 2020, 122, 109547.
- Wu, Z.Z.; Rao, M.; Xu, S.; Hu, H.Y.; Tang, Q.Z. Coumestrol ameliorates doxorubicin-induced cardiotoxicity via activating AMPKα. Free Radic. Res. 2020, 54, 629–639.
- Russo, M.; Guida, F.; Paparo, L.; Trinchese, G.; Aitoro, R.; Avagliano, C.; Fiordelisi, A.; Napolitano, F.; Mercurio, V.; Sala, V.; et al. The novel butyrate derivative phenylalanine-butyramide protects from doxorubicin-induced cardiotoxicity. Eur. J. Heart Fail. 2019, 21, 519–528.
- Shi, W.; Deng, H.; Zhang, J.; Zhang, Y.; Zhang, X.; Cui, G. Mitochondria-targeting small molecules effectively prevent cardiotoxicity induced by doxorubicin. Molecules 2018, 23, 1486.
- Jawa, Z.; Perez, R.M.; Garlie, L.; Singh, M.; Qamar, R.; Khandheria, B.K.; Jahangir, A.; Shi, Y. Risk factors of trastuzumab-induced cardiotoxicity in breast cancer: A meta-analysis. Medicine 2016, 95, 1–7.
- Cho, D.H.; Lim, I.R.; Kim, J.H.; Kim, M.N.; Kim, Y.H.; Park, K.H.; Park, S.M.; Shim, W.J. Protective effects of statin and angiotensin receptor blocker in a rat model of doxorubicin-and trastuzumab-induced cardiomyopathy. J. Am. Soc. Echocardiogr. 2020, 33, 1253–1263.
- Cuomo, A.; Rodolico, A.; Galdieri, A.; Russo, M.; Campi, G.; Franco, R.; Bruno, D.; Aran, L.; Carannante, A.; Attanasio, U.; et al. Heart failure and cancer: Mechanisms of old and new cardiotoxic drugs in cancer patients. Card. Fail. Rev. 2019, 5, 112–118.
- Saneeymehri, S.S.; Markey, K.R.; Mahipal, A. Paradoxical effect of capecitabine in 5-fluorouracil-induced cardiotoxicity: A case vignette and literature review. J. Oncol. Pharm. Pract. 2016, 22, 552–555.
- Shiga, T.; Hiraide, M. Cardiotoxicities of 5-Fluorouracil and other fluoropyrimidines. Curr. Treat. Options Oncol. 2020, 21, 1–21.
- Alter, P.; Herzum, M.; Soufi, M.; Schaefer, J.R.; Maisch, B. Cardiotoxicity of 5-fluorouracil. Cardiovasc. Hematol. Agents Med. Chem. 2006, 4, 1–5.
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med. Oncol. 2018, 10, 1–18.
- Charkviani, M.; Murvelashvili, N.; Barrera, F.; Sharma, A.; Eldin, R.S.; Nabil, N.U. Rare presentation of cardiotoxicity related to 5-Fluorouracil. Case Rep. Oncol. Med. 2020, 2020, 214.
- Dyhl-Polk, A.; Vaage-Nilsen, M.; Schou, M.; Vistisen, K.K.; Lund, C.M.; Kümler, T.; Appel, J.M.; Nielsen, D.L. Incidence and risk markers of 5-fluorouracil and capecitabine cardiotoxicity in patients with colorectal cancer. Acta Oncol. 2020, 59, 475–483.
- Nohria, A. The clinical conundrum of managing 5-fluorouracil–induced vasospasm in colorectal carcinoma. Cancer 2019, 125, 4346–4349.
- Mishra, T.; Shokr, M.; Ahmed, A.; Afonso, L. Acute reversible left ventricular systolic dysfunction associated with 5-fluorouracil therapy: A rare and increasingly recognised cardiotoxicity of a commonly used drug. BMJ Case Rep. CP 2019, 12, e230499.
- Muhammad, R.N.; Sallam, N.; El-Abhar, H.S. Activated ROCK/Akt/eNOS and ET-1/ERK pathways in 5-fluorouracil-induced cardiotoxicity: Modulation by simvastatin. Sci. Rep. 2020, 10, 1–4.
- Bayrak, S.; Aktaş, S.; Altun, Z.; Çakir, Y.; Tütüncü, M.; Kum Özşengezer, S.; Yilmaz, O.; Olgun, N. Antioxidant effect of acetyl-l-carnitine against cisplatin-induced cardiotoxicity. J. Int. Med. Res. 2020, 48, 1–11.
- Gunturk, E.E.; Yucel, B.; Gunturk, I.; Yazici, C.E.; Yay, A.R.; Kose, K. The effects of N-acetylcysteine on cisplatin induced cardiotoxicity. Bratislavskelekarskelisty 2019, 120, 423–428.
- Hu, Y.; Sun, B.; Zhao, B.; Mei, D.; Gu, Q.; Tian, Z. Cisplatin-induced cardiotoxicity with midrange ejection fraction: A case report and review of the literature. Medicine 2018, 97, e13807.
- Bahadır, A.; Ceyhan, A.; Gergin, Ö.Ö.; Yalçın, B.; Ülger, M.; Özyazgan, T.M.; Yay, A. Protective effects of curcumin and beta-carotene on cisplatin-induced cardiotoxicity: An experimental rat model. Anatol. J. Cardiol. 2018, 19, 213–221.
- Afsar, T.; Razak, S.; Almajwal, A.; Shabbir, M.; Khan, M.R. Evaluating the protective potency of Acacia hydaspica R. Parker on histological and biochemical changes induced by Cisplatin in the cardiac tissue of rats. BMC Complement. Altern. Med. 2019, 19, 1–2.
- Ibrahim, M.A.; Bakhaat, G.A.; Tammam, H.G.; Mohamed, R.M.; El-Naggar, S.A. Cardioprotective effect of green tea extract and vitamin E on Cisplatin-induced cardiotoxicity in mice: Toxicological, histological and immunohistochemical studies. Biomed. Pharmacother. 2019, 113, 108731.
- El-Hawwary, A.A.; Omar, N.M. The influence of ginger administration on cisplatin-induced cardiotoxicity in rat: Light and electron microscopic study. Actahistochemica 2019, 121, 553–562.
- Darby, S.C.; Cutter, D.J.; Boerma, M.; Constine, L.S.; Fajardo, L.F.; Kodama, K.; Mabuchi, K.; Marks, L.B.; Mettler, F.A.; Pierce, L.J.; et al. Radiation-related heart disease: Current knowledge and future prospects. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 656–665.
- Varricchi, G.; Galdiero, M.R.; Marone, G.; Criscuolo, G.; Triassi, M.; Bonaduce, D.; Marone, G.; Tocchetti, C.G. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2017, 2, 1–12.
- Dal’bo, N.; Patel, R.; Parikh, R.; Shah, S.P.; Guha, A.; Dani, S.S.; Ganatra, S. Cardiotoxicity of contemporary anticancer immunotherapy. Curr. Treat. Options Cardiovasc. Med. 2020, 22, 1–5.
- Ganatra, S.; Parikh, R.; Neilan, T.G. Cardiotoxicity of immune therapy. Cardiol. Clin. 2019, 37, 385–397.
- Upadhrasta, S.; Elias, H.; Patel, K.; Zheng, L. Managing cardiotoxicity associated with immune checkpoint inhibitors. Chronic Dis. Transl. Med. 2019, 5, 6–14.
- Lobenwein, D.; Kocher, F.; Dobner, S.; Gollmann-Tepeköylü, C.; Holfeld, J. Cardiotoxic mechanisms of cancer immunotherapy–A systematic review. Int. J. Cardiol. 2021, 323, 179–187.
- Brüstle, K.; Heidecker, B. Checkpoint inhibitor induced cardiotoxicity: Managing the drawbacks of our newest agents against cancer. Oncotarget 2017, 8, 106165–106166.
- Chung, W.B.; Youn, J.C.; Youn, H.J. Cardiovascular complications of novel anti-cancer immunotherapy: Old problems from new agents? Korean Circ. J. 2020, 50, 743–753.
- Heinzerling, L.; Ott, P.A.; Hodi, F.S.; Husain, A.N.; Tajmir-Riahi, A.; Tawbi, H.; Pauschinger, M.; Gajewski, T.F.; Lipson, E.J.; Luke, J.J. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J. Immunother. Cancer. 2016, 4, 1.
- Varricchi, G.; Marone, G.; Mercurio, V.; Galdiero, M.R.; Bonaduce, D.; Tocchetti, C.G. Immune checkpoint inhibitors and cardiac toxicity: An emerging issue. Curr. Med. Chem. 2018, 25, 1327–1339.
- Zarifa, A.; Albittar, A.; Kim, P.Y.; Hassan, S.; Palaskas, N.; Iliescu, C.; Durand, J.B.; Lopez-Mattei, J. Cardiac toxicities of anticancer treatments: Chemotherapy, targeted therapy and immunotherapy. Curr. Opin. Cardiol. 2019, 34, 441–450.
- Stein-Merlob, A.F.; Rothberg, M.V.; Holman, P.; Yang, E.H. Immunotherapy-associated cardiotoxicity of immune checkpoint inhibitors and chimeric antigen receptor T cell therapy: Diagnostic and management challenges and strategies. Curr. Cardiol. Rep. 2021, 23, 1.
- Ghosh, A.K.; Chen, D.H.; Guha, A.; Mackenzie, S.; Walker, J.M.; Roddie, C. CAR T cell therapy–related cardiovascular outcomes and management: Systemic disease or direct cardiotoxicity? Cardio Oncol. 2020, 2, 97–109.
- O’leary, B.; Finn, R.S.; Turner, N.C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 2016, 13, 417–430.
- Li, B.B.; Khan, N.; Ubellacker, J.M.; Xie, S.; Metzger-Filho, O.; Roberts, T.M.; Kim, H.; Mcallister, S.S.; Jean, J. CDK4/6 inhibition triggers anti-tumor immunity. Nature 2017, 548, 471–475.
- Goel, S.; DeCristo, M.J.; McAllister, S.S.; Zhao, J.J. CDK4/6 inhibition in cancer: Beyond cell cycle arrest. Trends Cell Biol. 2018, 28, 911–925.
- Scott, S.C.; Lee, S.S.; Abraham, J. Mechanisms of therapeutic CDK4/6 inhibition in breast cancer. Semin. Oncol. 2017, 44, 385–394.
- Martel, S.; Maurer, C.; Lambertini, M.; Pondé, N.; De Azambuja, E. Breast cancer treatment-induced cardiotoxicity. Expert Opin. Drug Saf. 2017, 16, 1021–1038.
- Santoni, M.; Occhipinti, G.; Romagnoli, E.; Miccini, F.; Scoccia, L.; Giulietti, M.; Principato, G.; Saladino, T.; Piva, F.; Battelli, N. Different cardiotoxicity of palbociclib and ribociclib in breast cancer: Gene expression and pharmacological data analyses, biological basis, and therapeutic implications. BioDrugs 2019, 33, 613–620.
- Itatani, Y.; Kawada, K.; Yamamoto, T.; Sakai, Y. Resistance to anti-angiogenic therapy in cancer—Alterations to anti-VEGF pathway. Int. J. Mol. Sci. 2018, 19, 1232.
- Lyons, T.G.; Ku, G.Y. Systemic therapy for esophagogastric cancer: Targeted therapies. Chin. Clin. Oncol. 2017, 6, 48–55.
- Cohen, A.C.; Roane, B.M.; Leath, C.A. Novel therapeutics for recurrent cervical cancer: Moving towards personalized therapy. Drugs 2020, 80, 217–227.
This entry is offline, you can click here to edit this entry!
