Hepatitis E virus (HEV), a pathogen that causes acute viral hepatitis, is a small icosahedral, quasi-enveloped, positive ssRNA virus. Its genome has three open reading frames (ORFs), with ORF1 and ORF3 encoding for nonstructural and regulatory proteins, respectively, while ORF2 is translated into the structural, capsid protein. ORF2 is most widely used for vaccine development in viral hepatitis. Hepatitis E virus-like particles (VLPs) are potential vaccine candidates against HEV infection. VLPs are composed of capsid subunits mimicking the natural configuration of the native virus but lack the genetic material needed for replication. As a result, VLPs are unable to replicate and cause disease, constituting safe vaccine platforms. Currently, the recombinant VLP-based vaccine Hecolin® against HEV is only licensed in China.
- Hepatitis E virus
- ORF2 capsid protein
- HEV VLPs
- vaccine
1. Introduction
2. Hepatitis E Genome Organization
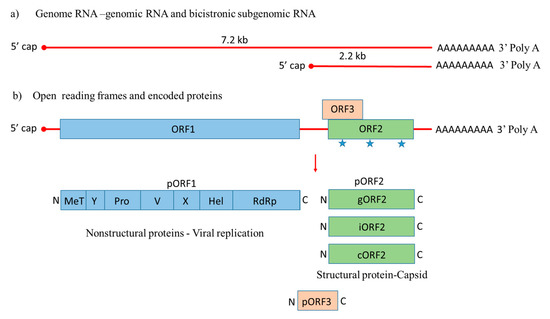
 ), with a small multifunctional protein encoded by ORF3. Three different capsid proteins have been discovered in vitro during infection, i.e., gORF2-glycosylated, iORF2-infectious and cORF2-cleaved ORF2.
), with a small multifunctional protein encoded by ORF3. Three different capsid proteins have been discovered in vitro during infection, i.e., gORF2-glycosylated, iORF2-infectious and cORF2-cleaved ORF2.References
- Kamar, N.; Bendall, R.; Legrand-Abravanel, F.; Xia, N.-S.; Ijaz, S.; Izopet, J.; Dalton, H.R. Hepatitis E. Lancet 2012, 379, 2477–2488. [Google Scholar] [CrossRef]
- Holla, R.P.; Ahmad, I.; Ahmad, Z.; Jameel, S. Molecular virology of hepatitis E virus. In Seminars in Liver Disease; Thieme Medical Publishers: New York, NY, USA, 2013; pp. 3–14. [Google Scholar]
- Meng, X.-J. From barnyard to food table: The omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 2011, 161, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Subhadra, S.; Singh, B.; Panda, B. Hepatitis E virus: The current scenario. Int. J. Infect. Dis. 2013, 17, e228–e233. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Thakral, D.; Rehman, S. Hepatitis E virus. Rev. Med Virol. 2007, 17, 151–180. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Teli, M.R.; Skidmore, S.; Sofi, M.A.; Khuroo, M.I. Incidence and severity of viral hepatitis in pregnancy. Am. J. Med. 1981, 70, 252–255. [Google Scholar] [CrossRef]
- Khuroo, M.; Kamili, S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J. Viral Hepat. 2003, 10, 61–69. [Google Scholar] [CrossRef]
- Jilani, N.; Das, B.C.; Husain, S.A.; Baweja, U.K.; Chattopadhya, D.; Gupta, R.K.; Sardana, S.; Kar, P. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J. Gastroenterol. Hepatol. 2007, 22, 676–682. [Google Scholar] [CrossRef]
- Allaire, M.; Bazille, C.; Selves, J.; Salamé, E.; Altieri, M. Hepatitis E virus infection mimicking acute graft rejection in a liver transplant recipient. Clin. Res. Hepatol. Gastroenterol. 2018, 42, e68–e71. [Google Scholar] [CrossRef]
- Behrendt, P.; Steinmann, E.; Manns, M.P.; Wedemeyer, H. The impact of hepatitis E in the liver transplant setting. J. Hepatol. 2014, 61, 1418–1429. [Google Scholar] [CrossRef]
- Marion, O.; Kamar, N. Hepatitis E Infections in Transplants. Emerg. Transpl. Infect. Clin. Chall. Implic. 2020, 1–18. [Google Scholar] [CrossRef]
- Pischke, S.; Peron, J.-M.; von Wulffen, M.; von Felden, J.; Höner zu Siederdissen, C.; Fournier, S.; Lütgehetmann, M.; Iking-Konert, C.; Bettinger, D.; Thimme, R. Chronic hepatitis e in rheumatology and internal medicine patients: A retrospective multicenter european cohort study. Viruses 2019, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Tavitian, S.; Peron, J.-M.; Huguet, F.; Kamar, N.; Abravanel, F.; Beyne-Rauzy, O.; Oberic, L.; Faguer, S.; Alric, L.; Roussel, M. Ribavirin for chronic hepatitis prevention among patients with hematologic malignancies. Emerg. Infect. Dis. 2015, 21, 1466. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Juarez, A.; Lopez-Lopez, P.; Frias, M.; Rivero, A. Hepatitis E infection in HIV-infected patients. Front. Microbiol. 2019, 10, 1425. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sharma, B.C.; Sarin, S.K. Hepatitis E virus as an etiology of acute exacerbation of previously unrecognized asymptomatic patients with hepatitis B virus-related chronic liver disease. J. Gastroenterol. Hepatol. 2008, 23, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Gérolami, R.; Moal, V.; Colson, P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N. Engl. J. Med. 2008, 358, 859–860. [Google Scholar] [CrossRef]
- Mclean, B.N.; Gulliver, J.; Dalton, H.R. Hepatitis E virus and neurological disorders. Pract. Neurol. 2017, 17, 282–288. [Google Scholar] [CrossRef]
- Abravanel, F.; Pique, J.; Couturier, E.; Nicot, F.; Dimeglio, C.; Lhomme, S.; Chiabrando, J.; Saune, K.; Péron, J.-M.; Kamar, N. Acute hepatitis E in French patients and neurological manifestations. J. Infect. 2018, 77, 220–226. [Google Scholar] [CrossRef]
- Kamar, N.; Weclawiak, H.; Guilbeau-Frugier, C.; Legrand-Abravanel, F.; Cointault, O.; Ribes, D.; Esposito, L.; Cardeau-Desangles, I.; Guitard, J.; Sallusto, F. Hepatitis E virus and the kidney in solid-organ transplant patients. Transplantation 2012, 93, 617–623. [Google Scholar] [CrossRef]
- Leaf, R.K.; O‘Brien, K.L.; Leaf, D.E.; Drews, R.E. Autoimmune hemolytic anemia in a young man with acute hepatitis E infection. Am. J. Hematol. 2017, 92, E77–E79. [Google Scholar] [CrossRef]
- Jaroszewicz, J.; Flisiak, R.; Kalinowska, A.; Wierzbicka, I.; Prokopowicz, D. Acute hepatitis E complicated by acute pancreatitis: A case report and literature review. Pancreas 2005, 30, 382–384. [Google Scholar] [CrossRef]
- Atsama, M.A.; Atangana, P.J.A.; Noah, D.N.; Moundipa, P.F.; Pineau, P.; Njouom, R. Hepatitis E virus infection as a promoting factor for hepatocellular carcinoma in Cameroon: Preliminary observations. Int. J. Infect. Dis. 2017, 64, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.-C.; Liu, C.-J.; Chang, C.T.; Su, T.-H.; Yang, W.-T.; Tsai, C.-H.; Chen, C.-L.; Yang, H.-C.; Liu, C.-H.; Chen, P.-J. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis. J. Hepatol. 2020, 72, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Nguyen, H.; Faulk, K.; Mather, K.; Torian, U.; Engle, R.; Emerson, S. Adaptation of a genotype 3 hepatitis E virus to efficient growth in cell culture depended on an inserted human gene segment acquired by recombination. J. Virol. 2012, 86, 5697–5707. [Google Scholar] [CrossRef] [PubMed]
- Schofield, D.; Glamann, J.; Emerson, S.; Purcell, R. Identification by phage display and characterization of two neutralizing chimpanzee monoclonal antibodies to the hepatitis E virus capsid protein. J. Virol. 2000, 74, 5548–5555. [Google Scholar] [CrossRef]
- Roldão, A.; Mellado, M.C.M.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef] [PubMed]
- Noad, R.; Roy, P. Virus-like particles as immunogens. Trends Microbiol. 2003, 11, 438–444. [Google Scholar] [CrossRef]
- Grgacic, E.V.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods 2006, 40, 60–65. [Google Scholar] [CrossRef]
- Cox, R.G.; Erickson, J.J.; Hastings, A.K.; Becker, J.C.; Johnson, M.; Craven, R.E.; Tollefson, S.J.; Boyd, K.L.; Williams, J.V. Human metapneumovirus virus-like particles induce protective B and T cell responses in a mouse model. J. Virol. 2014, 88, 6368–6379. [Google Scholar] [CrossRef]
- Rts, S.; Agnandji, S.T.; Lell, B.; Fernandes, J.F.; Abossolo, B.P.; Methogo, B.; Kabwende, A.L.; Adegnika, A.A.; Mordmueller, B.; Issifou, S. A phase 3 trial of RTS, S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 2012, 367, 2284–2295. [Google Scholar]
- Scotti, N.; Rybicki, E.P. Virus-like particles produced in plants as potential vaccines. Expert Rev. Vaccines 2013, 12, 211–224. [Google Scholar] [CrossRef]
- Almeida, J.; Edwards, D.C.; Brand, C.; Heath, T. Formation of virosomes from influenza subunits and liposomes. Lancet 1975, 306, 899–901. [Google Scholar] [CrossRef]
- Keating, G.M.; Noble, S. Recombinant hepatitis B vaccine (Engerix-B®). Drugs 2003, 63, 1021–1051. [Google Scholar] [CrossRef] [PubMed]
- Monie, A.; Hung, C.-F.; Roden, R.; Wu, T.C. Cervarix™: A vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biol. Targets Ther. 2008, 2, 107. [Google Scholar]
- Venters, C.; Graham, W.; Cassidy, W. Recombivax-HB: Perspectives past, present and future. Expert Rev. Vaccines 2004, 3, 119–129. [Google Scholar] [CrossRef]
- Shi, L.; Sings, H.; Bryan, J.; Wang, B.; Wang, Y.; Mach, H.; Kosinski, M.; Washabaugh, M.; Sitrin, R.; Barr, E. GARDASIL®: Prophylactic human papillomavirus vaccine development–from bench top to bed-side. Clin. Pharmacol. Ther. 2007, 81, 259–264. [Google Scholar] [CrossRef]
- Crevar, C.J.; Ross, T.M. Elicitation of protective immune responses using a bivalent H5N1 VLP vaccine. Virol. J. 2008, 5, 1. [Google Scholar] [CrossRef]
- Treanor, J.J.; Atmar, R.L.; Frey, S.E.; Gormley, R.; Chen, W.H.; Ferreira, J.; Goodwin, R.; Borkowski, A.; Clemens, R.; Mendelman, P.M. A novel intramuscular bivalent norovirus virus-like particle vaccine candidate—Reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J. Infect. Dis. 2014, 210, 1763–1771. [Google Scholar] [CrossRef]
- Smith, D.B.; Simmonds, P.; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.-J.; Okamoto, H.; Van der Poel, W.H.; Purdy, M.A.; Members of the International Committee on the Taxonomy of Viruses Hepeviridae Study Group. Consensus proposals for classification of the family Hepeviridae. J. Gen. Virol. 2014, 95, 2223–2232. [Google Scholar] [CrossRef]
- Takahashi, M.; Nishizawa, T.; Nagashima, S.; Jirintai, S.; Kawakami, M.; Sonoda, Y.; Suzuki, T.; Yamamoto, S.; Shigemoto, K.; Ashida, K. Molecular characterization of a novel hepatitis E virus (HEV) strain obtained from a wild boar in Japan that is highly divergent from the previously recognized HEV strains. Virus Res. 2014, 180, 59–69. [Google Scholar] [CrossRef]
- Woo, P.; Lau, S.; Teng, J.; Tsang, A.; Joseph, M.; Wong, E.; Tang, Y.; Sivakumar, S.; Xie, J.; Bai, R. New hepatitis E virus genotype in camels, the Middle East. Emerg. Infect. Dis. 2014, 20, e8. [Google Scholar] [CrossRef]
- Reyes, G.R.; Purdy, M.A.; Kim, J.P.; Ka-Cheung, L.; Young, L.M. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science 1990, 247, 1335. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.W.; Smith, M.M.; Guerra, M.E.; Huang, C.-C.; Bradley, D.W.; Fry, K.E.; Reyes, G.R. Hepatitis E virus (HEV): Molecular cloning and sequencing of the full-length viral genome. Virology 1991, 185, 120–131. [Google Scholar] [CrossRef]
- Graff, J.; Torian, U.; Nguyen, H.; Emerson, S.U. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J. Virol. 2006, 80, 5919–5926. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Holla, R.P.; Jameel, S. Molecular virology of hepatitis E virus. Virus Res. 2011, 161, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Meng, X.-J. Molecular biology and replication of hepatitis E virus. Emerg. Microbes Infect. 2012, 1, e17. [Google Scholar] [CrossRef] [PubMed]
- Kannan, H.; Fan, S.; Patel, D.; Bossis, I.; Zhang, Y.-J. The hepatitis E virus open reading frame 3 product interacts with microtubules and interferes with their dynamics. J. Virol. 2009, 83, 6375–6382. [Google Scholar] [CrossRef]
- Ding, Q.; Heller, B.; Capuccino, J.M.; Song, B.; Nimgaonkar, I.; Hrebikova, G.; Contreras, J.E.; Ploss, A. Hepatitis E virus ORF3 is a functional ion channel required for release of infectious particles. Proc. Natl. Acad. Sci. USA 2017, 114, 1147–1152. [Google Scholar] [CrossRef]
- Jameel, S.; Zafrullah, M.; Ozdener, M.H.; Panda, S.K. Expression in animal cells and characterization of the hepatitis E virus structural proteins. J. Virol. 1996, 70, 207–216. [Google Scholar] [CrossRef]
- He, S.; Miao, J.; Zheng, Z.; Wu, T.; Xie, M.; Tang, M.; Zhang, J.; Ng, M.-H.; Xia, N. Putative receptor-binding sites of hepatitis E virus. J. Gen. Virol. 2008, 89, 245–249. [Google Scholar] [CrossRef]
- Kalia, M.; Chandra, V.; Rahman, S.A.; Sehgal, D.; Jameel, S. Heparan sulfate proteoglycans are required for cellular binding of the hepatitis E virus ORF2 capsid protein and for viral infection. J. Virol. 2009, 83, 12714–12724. [Google Scholar] [CrossRef]
- Xing, L.; Wang, J.C.; Li, T.-C.; Yasutomi, Y.; Lara, J.; Khudyakov, Y.; Schofield, D.; Emerson, S.U.; Purcell, R.H.; Takeda, N. Spatial configuration of hepatitis E virus antigenic domain. J. Virol. 2011, 85, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Torresi, J.; Li, F.; Locarnini, S.A.; Anderson, D.A. Only the non-glycosylated fraction of hepatitis E virus capsid (open reading frame 2) protein is stable in mammalian cells. J. Gen. Virol. 1999, 80, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Kato, K.; Li, T.; Takeda, N.; Miyamura, T.; Hammar, L.; Cheng, R.H. Recombinant hepatitis E capsid protein self-assembles into a dual-domain T= 1 particle presenting native virus epitopes. Virology 1999, 265, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Guu, T.S.; Liu, Z.; Ye, Q.; Mata, D.A.; Li, K.; Yin, C.; Zhang, J.; Tao, Y.J. Structure of the hepatitis E virus-like particle suggests mechanisms for virus assembly and receptor binding. Proc. Natl. Acad. Sci. USA 2009, 106, 12992–12997. [Google Scholar] [CrossRef]
- Xing, L.; Li, T.-C.; Mayazaki, N.; Simon, M.N.; Wall, J.S.; Moore, M.; Wang, C.-Y.; Takeda, N.; Wakita, T.; Miyamura, T. Structure of hepatitis E virion-sized particle reveals an RNA-dependent viral assembly pathway. J. Biol. Chem. 2010, 285, 33175–33183. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, F.; Zhang, L.; Harrison, T.J.; Huang, W.; Zhao, C.; Kong, W.; Jiang, C.; Wang, Y. Hepatitis E virus produced from cell culture has a lipid envelope. PLoS ONE 2015, 10, e0132503. [Google Scholar] [CrossRef]
- Yin, X.; Ying, D.; Lhomme, S.; Tang, Z.; Walker, C.M.; Xia, N.; Zheng, Z.; Feng, Z. Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection. Proc. Natl. Acad. Sci. USA 2018, 115, 4773–4778. [Google Scholar] [CrossRef]
- Montpellier, C.; Wychowski, C.; Sayed, I.M.; Meunier, J.-C.; Saliou, J.-M.; Ankavay, M.; Bull, A.; Pillez, A.; Abravanel, F.; Helle, F. Hepatitis E virus lifecycle and identification of 3 forms of the ORF2 capsid protein. Gastroenterology 2018, 154, 211–223. [Google Scholar] [CrossRef]
- Fu, R.M.; Decker, C.C.; Dao Thi, V.L. Cell culture models for hepatitis E virus. Viruses 2019, 11, 608. [Google Scholar] [CrossRef]
- Rogee, S.; Talbot, N.; Caperna, T.; Bouquet, J.M.; Barnaud, E.; Pavio, N. New models of hepatitis E virus replication in human and porcine hepatocyte cell lines. J. Gen. Virol. 2013, 94, 549–558. [Google Scholar] [CrossRef]
- Capelli, N.; Marion, O.; Dubois, M.; Allart, S.; Bertrand-Michel, J.; Lhomme, S.; Abravanel, F.; Izopet, J.; Chapuy-Regaud, S. Vectorial release of hepatitis E virus in polarized human hepatocytes. J. Virol. 2019, 93, 93. [Google Scholar] [CrossRef] [PubMed]
- Capelli, N.; Dubois, M.; Pucelle, M.; Da Silva, I.; Lhomme, S.; Abravanel, F.; Chapuy-Regaud, S.; Izopet, J. Optimized hepatitis E virus (HEV) culture and its application to measurements of HEV infectivity. Viruses 2020, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Thi, V.L.D.; Wu, X.; Belote, R.L.; Andreo, U.; Takacs, C.N.; Fernandez, J.P.; Vale-Silva, L.A.; Prallet, S.; Decker, C.C.; Fu, R.M. Stem cell-derived polarized hepatocytes. Nat. Commun. 2020, 11, 1–13. [Google Scholar]
- Kamar, N.; Marion, O.; Abravanel, F.; Izopet, J.; Dalton, H.R. Extrahepatic manifestations of hepatitis E virus. Liver Int. 2016, 36, 467–472. [Google Scholar] [CrossRef]
This entry is adapted from the peer-reviewed paper 10.3390/v12080826
