The specificity of a diagnostic assay depends upon the purity of the biomolecules used as a probe. To get specific and accurate information of a disease, the use of synthetic peptides in diagnostics have increased in the last few decades, because of their high purity profile and ability to get modified chemically. The discovered peptide probes are used either in imaging diagnostics or in non-imaging diagnostics. In non-imaging diagnostics, techniques such as Enzyme-Linked Immunosorbent Assay (ELISA), lateral flow devices (i.e., point-of-care testing), or microarray or LC-MS/MS are used for direct analysis of biofluids. Among all, peptide-based ELISA is considered to be the most preferred technology platform. Similarly, peptides can also be used as probes for imaging techniques, such as single-photon emission computed tomography (SPECT) and positron emission tomography (PET). The role of radiolabeled peptides, such as somatostatin receptors, interleukin 2 receptor, prostate specific membrane antigen, αβ3 integrin receptor, gastrin-releasing peptide, chemokine receptor 4, and urokinase-type plasminogen receptor, are well established tools for targeted molecular imaging ortumor receptor imaging. Low molecular weight peptides allow a rapid clearance from the blood and result in favorable target-to-non-target ratios. It also displays a good tissue penetration and non-immunogenicity. The only drawback of using peptides is their potential low metabolic stability.
- peptides
- diagnostic
- molecular imaging
- PET imaging
- Nuclear Medicine
1. Role of Peptides in Diagnostics
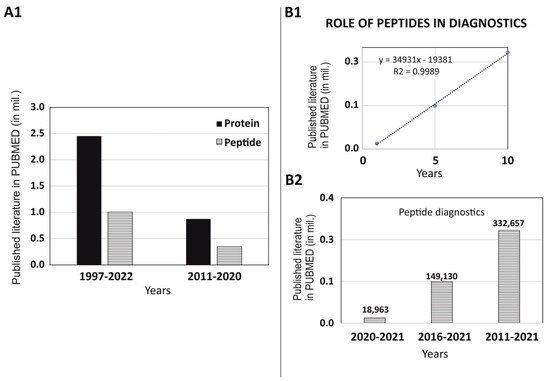
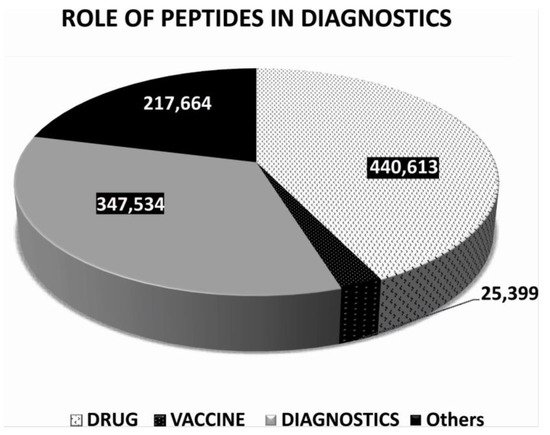
2. Non-Imaging Diagnostics
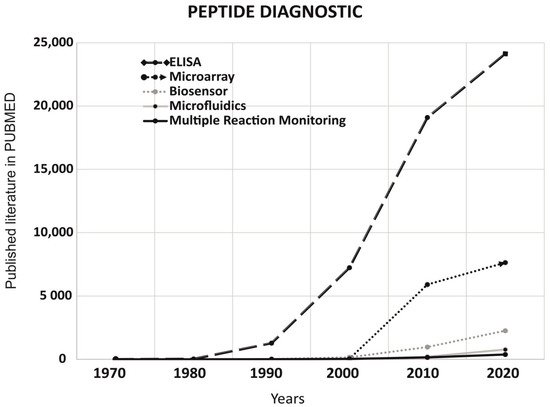
3. Peptides Application in Non-Imaging Diagnostics
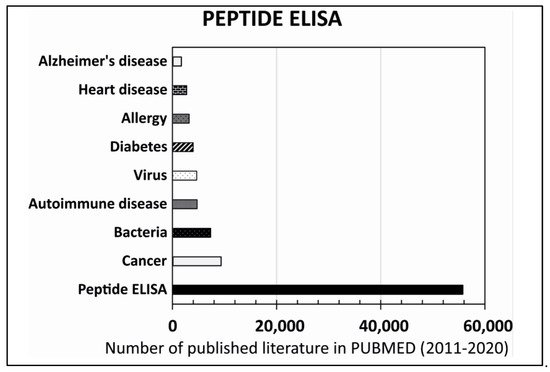
This entry is adapted from the peer-reviewed paper 10.3390/ijms22168828
References
- Brown, L.; Westby, M.; Souberbielle, B.E.; Szawlowski, P.W.; Kemp, G.; Hay, P.; Dalgleish, A.G. Optimisation of a peptide-based indirect ELISA for the detection of antibody in the serum of HIV-1 seropositive patients. J. Immunol. Methods 1997, 200, 79–88.
- Gresch, S.C.; Mutch, L.A.; Janecek, J.L.; Hegstad-Davies, R.L.; Graham, M.L. Cross-validation of commercial enzyme-linked immunosorbent assay and radioimmunoassay for porcine C-peptide concentration measurements in non-human primate serum. Xenotransplantation 2017, 24, e12320.
- Graham, M.L.; Gresch, S.C.; Hardy, S.K.; Mutch, L.A.; Janecek, J.L.; Hegstad-Davies, R.L. Evaluation of commercial ELISA and RIA for measuring porcine C-peptide: Implications for research. Xenotransplantation 2015, 22, 62–69.
- Lau, M.S.; Mooney, P.D.; White, W.L.; Rees, M.A.; Wong, S.H.; Hadjivassiliou, M.; Green, P.H.R.; Lebwohl, B.; Sanders, D.S. Office-Based Point of Care Testing (IgA/IgG-Deamidated Gliadin Peptide) for Celiac Disease. Am. J. Gastroenterol. 2018, 113, 1238–1246.
- Liu, M.; Zhao, G.; Wei, B.F. Attenuated serum vasoactive intestinal peptide concentrations are correlated with disease severity of non-traumatic osteonecrosis of femoral head. J. Orthop. Surg. Res. 2021, 16, 325.
- De-Simone, S.G.; Gomes, L.R.; Napoleão-Pêgo, P.; Lechuga, G.C.; de Pina, J.S.; Epitope, F.R.D. Mapping of the Diphtheria Toxin and Development of an ELISA-Specific Diagnostic Assay. Vaccines 2021, 9, 313.
- Gupta, K.; Brown, L.; Bakshi, R.K.; Press, C.G.; Chi, X.; Gorwitz, R.J.; Papp, J.R.; Geisler, W.M. Performance of Chlamydia trachomatis OmcB Enzyme-Linked Immunosorbent Assay in Serodiagnosis of Chlamydia trachomatis Infection in Women. J. Clin. Microbiol. 2018, 56, e00275-18.
- Rahman, K.S.; Darville, T.; Russell, A.N.; O’Connell, C.M.; Wiesenfeld, H.C.; Hillier, S.L.; Chowdhury, E.U.; Juan, Y.C.; Kaltenboeck, B. Discovery of Human-Specific Immunodominant Chlamydia trachomatis B Cell Epitopes. Msphere 2018, 3, e00246-18.
- Rahman, K.S.; Darville, T.; Russell, A.N.; O’Connell, C.M.; Wiesenfeld, H.C.; Hillier, S.L.; Lee, D.E.; Kaltenboeck, B. Comprehensive Molecular Serology of Human Chlamydia trachomatis Infections by Peptide Enzyme-Linked Immunosorbent Assays. Msphere 2018, 3, e00253-18.
- Rahman, K.S.; Darville, T.; Wiesenfeld, H.C.; Hillier, S.L.; Kaltenboeck, B. Mixed Chlamydia trachomatis Peptide Antigens Provide a Specific and Sensitive Single-Well Colorimetric Enzyme-Linked Immunosorbent Assay for Detection of Human Anti-C. trachomatis Antibodies. Msphere 2018, 3, e00484-18.
- Van Kruiningen, H.J.; Helal, Z.; Leroyer, A.; Garmendia, A.; Gower-Rousseau, C. ELISA Serology for Antibodies Against Chlamydia trachomatis in Crohn’s Disease. Gastroenterol. Res. 2017, 10, 334–338.
- Mosadeghi, P.; Heydari-Zarnagh, H. Development and Evaluation of a Novel ELISA for Detection of Antibodies against HTLV-I Using Chimeric Peptides. Iran. J. Allergy Asthma Immunol. 2018, 17, 144–150.
- Li, Y.; Lai, D.Y.; Lei, Q.; Xu, Z.W.; Wang, F.; Hou, H.; Chen, L.; Wu, J.; Ren, Y.; Ma, M.L.; et al. Systematic evaluation of IgG responses to SARS-CoV-2 spike protein-derived peptides for monitoring COVID-19 patients. Cell Mol. Immunol. 2021, 18, 621–631.
- Liu, J.; Cui, D.; Jiang, Y.; Li, Y.; Liu, Z.; Tao, L.; Zhao, Q.; Diao, A. Selection and characterization of a novel affibody peptide and its application in a two-site ELISA for the detection of cancer biomarker alpha-fetoprotein. Int. J. Biol. Macromol. 2021, 166, 884–892.
- Sahin, D.; Taflan, S.O.; Yartas, G.; Ashktorab, H.; Smoot, D.T. Screening and Identification of Peptides Specifically Targeted to Gastric Cancer Cells from a Phage Display Peptide Library. Asian Pac. J. Cancer Prev. 2018, 19, 927–932.
- Liu, Y.; Xia, X.; Wang, Y.; Li, X.; Zhou, G.; Liang, H.; Feng, G.; Zheng, C. Screening and identification of a specific peptide for targeting hypoxic hepatoma cells. Mol. Cell Probes 2016, 30, 246–253.
- Zhang, W.J.; Sui, Y.X.; Budha, A.; Zheng, J.B.; Sun, X.J.; Hou, Y.C.; Wang, T.D.; Lu, S.Y. Affinity peptide developed by phage display selection for targeting gastric cancer. World J. Gastroenterol. 2012, 18, 2053–2060.
- Galvis-Jiménez, J.M.; Curtidor, H.; Patarroyo, M.A.; Monterrey, P.; Ramírez-Clavijo, S.R. Mammaglobin peptide as a novel biomarker for breast cancer detection. Cancer Biol. Ther. 2013, 14, 327–332.
- Wettergren, A.; Wøjdemann, M.; Holst, J.J. The inhibitory effect of glucagon-like peptide-1 (7-36)amide on antral motility is antagonized by its N-terminally truncated primary metabolite GLP-1 (9-36)amide. Peptides 1998, 19, 877–882.
- Wewer Albrechtsen, N.J.; Asmar, A.; Jensen, F.; Törang, S.; Simonsen, L.; Kuhre, R.E.; Asmar, M.; Veedfald, S.; Plamboeck, A.; Knop, F.K.; et al. A sandwich ELISA for measurement of the primary glucagon-like peptide-1 metabolite. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E284–E291.
- Wewer Albrechtsen, N.J.; Bak, M.J.; Hartmann, B.; Christensen, L.W.; Kuhre, R.E.; Deacon, C.F.; Holst, J.J. Stability of glucagon-like peptide 1 and glucagon in human plasma. Endocr. Connect 2015, 4, 50–57.
- Wenzel, K.; Schulze-Rothe, S.; Müller, J.; Wallukat, G.; Haberland, A. Difference between beta1-adrenoceptor autoantibodies of human and animal origin-Limitations detecting beta1-adrenoceptor autoantibodies using peptide based ELISA technology. PLoS ONE 2018, 13, e0192615.
- Lv, R.; Chen, Y.; Xia, N.; Liang, Y.; He, Q.; Li, M.; Qi, Z.; Lu, Y.; Zhao, S. Development of a double-antibody sandwich ELISA for rapid detection to C-peptide in human urine. J. Pharm. Biomed. Anal. 2019, 162, 179–184.
- Velumani, S.; Ho, H.T.; He, F.; Musthaq, S.; Prabakaran, M.; Kwang, J. A novel peptide ELISA for universal detection of antibodies to human H5N1 influenza viruses. PLoS ONE 2011, 6, e20737.
- Tiwari, R.P.; Jain, A.; Khan, Z.; Kumar, P.; Bhrigu, V.; Bisen, P.S. Designing of novel antigenic peptide cocktail for the detection of antibodies to HIV-1/2 by ELISA. J. Immunol. Methods 2013, 387, 157–166.
- Van Burgel, N.D.; Brandenburg, A.; Gerritsen, H.J.; Kroes, A.C.; van Dam, A.P. High sensitivity and specificity of the C6-peptide ELISA on cerebrospinal fluid in Lyme neuroborreliosis patients. Clin. Microbiol. Infect. 2011, 17, 1495–1500.
- Davis, J.B. ELISA for Monitoring Nerve Growth Factor. Methods Mol. Biol. 2017, 1606, 141–147.
