Biomolecular condensates are membraneless organelles (MLOs) that form dynamic, chemically distinct subcellular compartments organizing macromolecules such as proteins, RNA, and DNA in unicellular prokaryotic bacteria and complex eukaryotic cells. Separated from surrounding environments, MLOs in the nucleoplasm, cytoplasm, and mitochondria assemble by liquid–liquid phase separation (LLPS) into transient, non-static, liquid-like droplets that regulate essential molecular functions. LLPS is primarily controlled by ATP-dependent post-translational modifications (PTMs) that fine-tune the balance between attractive and repulsive charge states and/or binding motifs of proteins. Aberrant phase separation due to the absence of adequate hydrotropic small molecules such as ATP can cause pathological protein aggregation in diseases such as neurodegenerative disorders. Melatonin is a potent antioxidant capable of protecting cardiolipin and membrane lipids raft domains from peroxidation to support ATPase functionality and ion channel activities that may exert a dominant influence over phase separation in biomolecular condensates during condensate coacervation or dissolution processes that are ATP-dependent.
- melatonin
- biomolecular condensate
- neurodegenerative disorder
- liquid–liquid phase separation
- ATP
- lipid raft
- post-translational modification
- m6A
- RNA
1. Introduction
2. ATP Regulates Biomolecular Condensates
2.1. Dimerized ATP Synthase/ATPase Require High-Curvature Lipid Domains
2.2. Translocation of ATP Dimers to Lipid Rafts Are Cellular Responses to Stress and Stimuli
Biomolecular condensates adapt to changing endogenous or exogenous conditions [3] by continuously fine-tuning biochemical reactions, enriching or excluding biomolecules from their environment [7]. The rapid translocation of mitochondrial ATP synthase to lipid rafts may be integral to these adaptive responses because ATP functions not only as a biological hydrotrope [30][178], increasing the solubility of positively charged, intrinsically disordered proteins [179], but may act as a universal and specific regulator of intrinsically disordered regions (IDRs) capable of altering physicochemical properties, conformation dynamics, assembly, and aggregation [45], in addition to providing phosphates as an energy source to fuel post-translational modifications that regulate the fluctuation of biomolecule phase separation during condensate formation [79][178]. Failure to maintain nanoscopic lipid raft domains with appropriate line tension and membrane elasticity [185] to functionally host dimerized ATPase [186], ATP synthase [175] may contribute to aberrant phase separation, resulting in pathogenic protein aggregates in neurodegeneration [11] and cancer [10][12].
The ability of ATP synthase/ATPase to form dimerized rows on the IMM of mitochondria and other membrane surfaces may be highly dependent upon membrane lipid composition [211] and curvature [175]. Uncontrolled, excess oxidative stress can cause lipid peroxidation [212] which induces pathological changes to membrane lipid composition, including alterations of cardiolipin in IMMs [211][213], as well as changes in membrane curvature that prevent optimal dimerization and the subsequent functioning of ATP synthase/ATPase [214][215]. Insufficient or depletion of ATP can directly impact the physical and functional properties of biomolecular condensates [32][33][79][118]. ATP is not only a biological hydrotrope capable of inhibiting protein LLPS and aggregation at high mM concentrations; it has recently been observed to act as a universal and specific regulator of IDRs, altering their physicochemical properties, conformation dynamics, assembly, and aggregation [45].
3. Melatonin Is a Potent Ancient Antioxidant That Protects ATP Levels to Regulate the Formation and Dissolution of MLOs
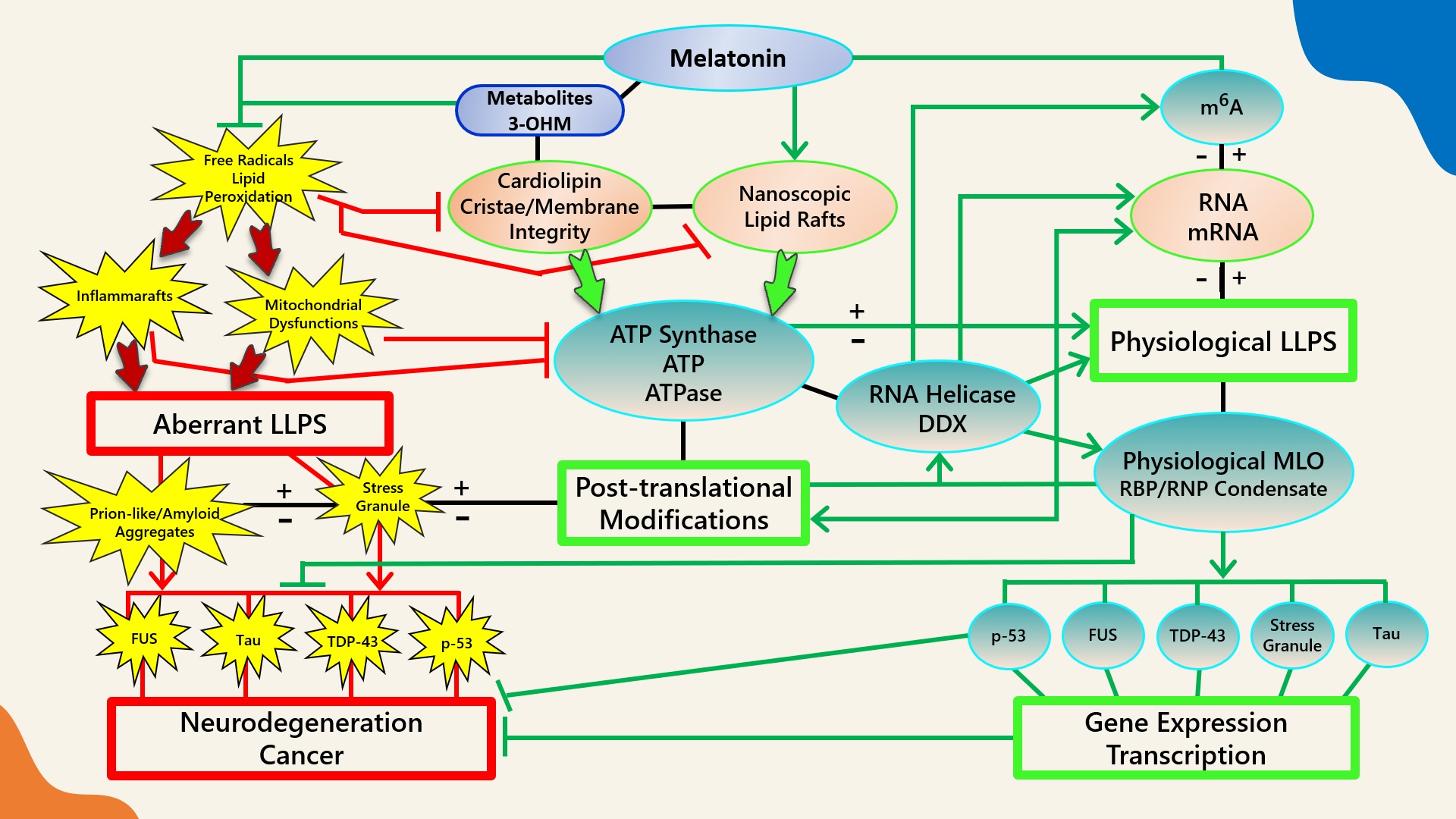 Figure 1. Schematic illustrating the regulation of biomolecular condensates by melatonin represented through observations reported in antioxidant protection against lipid peroxidation to maintain membrane/lipid raft composition/stability that serves to maintain adequate ATP levels in all cellular compartments to fuel, support, and regulate post-translational/m6A modifications that may fine-tune RNA dynamics in the assembly and disassembly of MLOs to prevent pathological aggregations in neurodegenerative disorders. LLPS: liquid–liquid phase separation; DDX: Dead-box RNA helicase; m6A: N6-methyladenosine; MLO: membraneless organelle; RBP: RNA-binding protein; RNP: ribonucleoprotein; PTM: post-translational modification (See Abbreviations for additional acronyms).
Figure 1. Schematic illustrating the regulation of biomolecular condensates by melatonin represented through observations reported in antioxidant protection against lipid peroxidation to maintain membrane/lipid raft composition/stability that serves to maintain adequate ATP levels in all cellular compartments to fuel, support, and regulate post-translational/m6A modifications that may fine-tune RNA dynamics in the assembly and disassembly of MLOs to prevent pathological aggregations in neurodegenerative disorders. LLPS: liquid–liquid phase separation; DDX: Dead-box RNA helicase; m6A: N6-methyladenosine; MLO: membraneless organelle; RBP: RNA-binding protein; RNP: ribonucleoprotein; PTM: post-translational modification (See Abbreviations for additional acronyms).3.1. Melatonin Metabolite 3-OHM Inhibits Lipid Peroxidation by Hydroperoxyl Radical
3.2. Melatonin Is Preferentially Located at Hydrophilic/Hydrophobic Membrane Interfaces
3.3. Melatonin Metabolite Free Radical Scavenging Cascades Rescue Cardiolipin from Hydroperoxyl Radicals (•OOH)
3.4. Melatonin Antioxidant Cascades May Inhibit NLRP3 Prionoid-Like Aggregation in an ATP-Dependent Manner
Cardiolipin (CL) is a mitochondria signature lipid distinctly attracted to membrane lipid domains with strong negative curvatures, such as the apex of IMM cristae [226][228]. CL is often externalized to the outer mitochondrial membrane (OMM) upon mitochondrial distress from ROS attacks [258][259], whereas oxidized CL in OMM initiates apoptotic signaling processes [260] that can lead to opening of the mitochondrial permeability transition pore (mPTP) and the release of cytochrome c (Cyt c) [261][262]. Externalized CL, whether oxidized or not, becomes an essential signaling platform that binds and interacts with important mitophagic, autophagic, and inflammatory enzymes [259][263], including Beclin 1 [264], tBid, Bax [262][265], caspase-8 [266], and the NLR pyrin domain containing 3 (NLRP3) inflammasomes [267]. A major source of extremely inflammatory cytokines IL-1β and IL-18 [268], NLRP3 inflammasome is a phase-separated supramolecular complex that mediates immune responses upon the detection of cellular stress and dysfunction [269][270][271]. The activation of the NLRP3 inflammasome in macrophages is induced by oxidized phospholipids [272], whereas the docking of externalized CL to NLRP3 inflammasome primes its assembly and subsequent activation in mitochondria [267] as well as mitochondria-associated membranes (MAMs), a region comprising highly specialized proteins which is tethered to the endoplasmic reticulum (ER) [273][274].
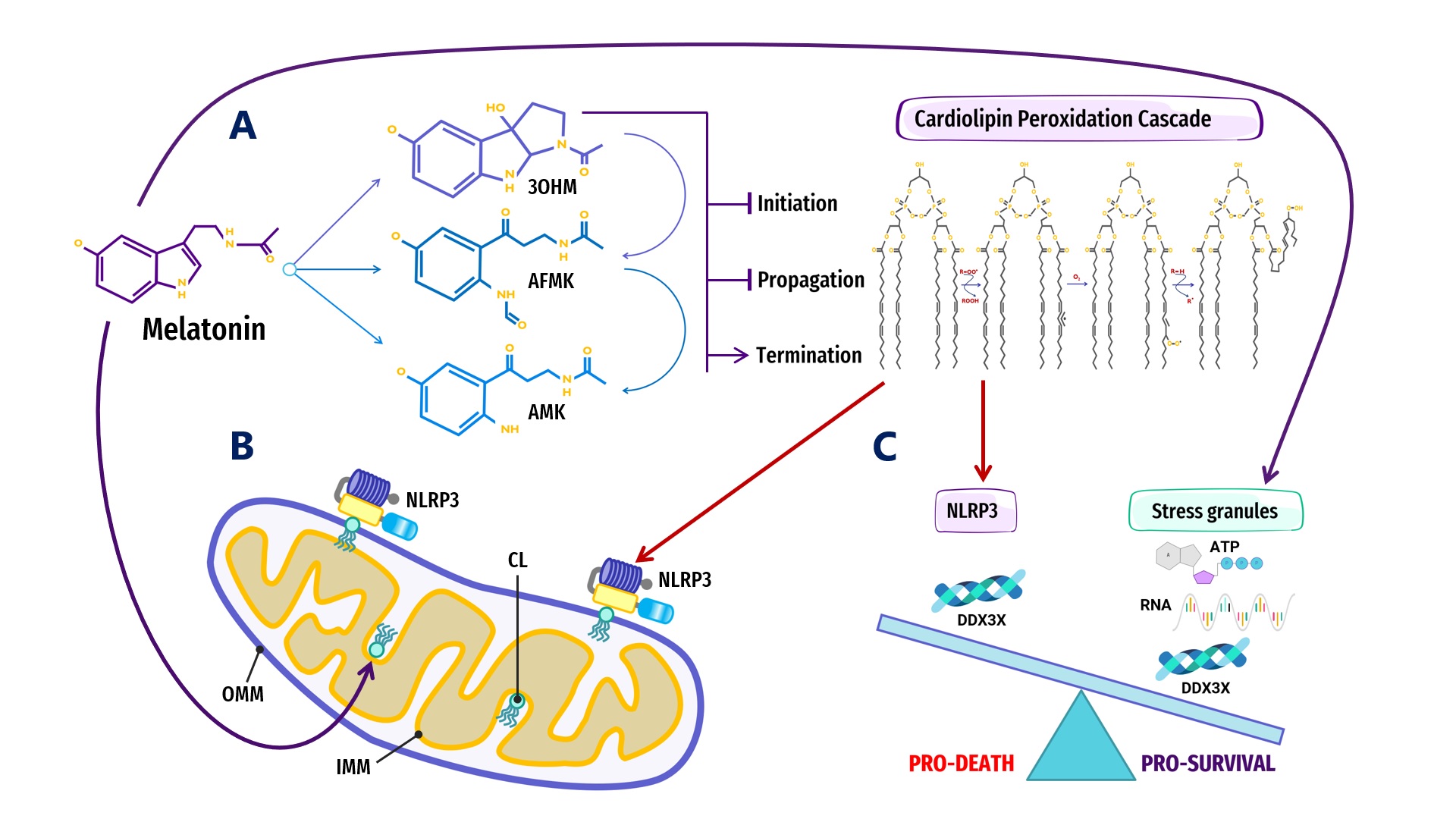 Figure 2. Overview of melatonin regulation of NLRP3 inflammasome (NLRP3) formation, assembly and activation: (A) Summary of melatonin and metabolite antioxidant cascade inhibiting the initiation and propagation of cardiolipin (CL) peroxidation, effectively terminating the CL peroxidation cascade; (B) Oxidized CL is externalized from the cristae/inner mitochondrial membrane (IMM) to the outer mitochondrial membrane (OMM) where it docks and primes NLRP3 inflammasome assembly prior to activation in mitochondria; (C) DDX3X, an ATP-dependent DEAD-box RNA helicase, is the mediator that selects the formation of “Pro-Survival” stress granules or the transition of the NLRP3 inflammasome into “Pro-Death”, stable, prionoid-like complexes. The successful formation of stress granules is also dependent upon the availability of ATP and RNA, both of which may be regulated by melatonin (See Abbreviations for additional acronyms).
Figure 2. Overview of melatonin regulation of NLRP3 inflammasome (NLRP3) formation, assembly and activation: (A) Summary of melatonin and metabolite antioxidant cascade inhibiting the initiation and propagation of cardiolipin (CL) peroxidation, effectively terminating the CL peroxidation cascade; (B) Oxidized CL is externalized from the cristae/inner mitochondrial membrane (IMM) to the outer mitochondrial membrane (OMM) where it docks and primes NLRP3 inflammasome assembly prior to activation in mitochondria; (C) DDX3X, an ATP-dependent DEAD-box RNA helicase, is the mediator that selects the formation of “Pro-Survival” stress granules or the transition of the NLRP3 inflammasome into “Pro-Death”, stable, prionoid-like complexes. The successful formation of stress granules is also dependent upon the availability of ATP and RNA, both of which may be regulated by melatonin (See Abbreviations for additional acronyms).ATP-dependent DEAD-box RNA helicases (DDXs) are ATPases that post-translationally regulate RNA-containing phase-separated organelles in prokaryotes and eukaryotes [307][308]. DDXs promote phase separation in their ATP-bound form, but can also release RNA and induce compartment turnover using ATP hydrolysis. Inhibition of DDX ATPase activity can disrupt the disassembly of physiological MLOs such as P-bodies and stress granules [69][309] (Figure 1). Phosphorylation is one of the most important PTMs that can control the assembly/disassembly of MLOs [608] as well as stabilize or destabilize MLOs including G bodies [512] and p53 [609]. Cells rely on phosphorylation as rapid, reversible responses to different stimuli by changing the physicochemical properties of proteins during phase separation multivalent interactions [79][538]. Phosphorylation establishes covalent bonds between phosphoryl and amino acid hydroxyl groups using the terminal phosphate group in ATP [610]. The ATP-dependent DEAD-box helicase [307] DDX3X responsible for initiating NLRP3 inflammasome aggregation is dependent upon phosphorylation-associated IFN promoter stimulation [304][310][615][616]. When the conserved, eukaryotic, integrated stress response (ISR) pathway is activated by external stress stimuli including hypoxia, nutrient deprivation, viral infections, as well as intrinsic ER stress [617], the phosphorylation of eukaryotic translation initiation factor 2 alpha (eIF2a) on Ser51 [618][619] triggers the formation of stress granules as adaptive homeostatic responses to promote survival and restore homeostasis [620][621][622][623]. It is presently unknown what prompts DDX3X to select the aggregation of pro-survival stress granules over pro-death NLRP3 inflammasomes or vice versa [304][310]. It would not be unreasonable to assume that an excessive oxidative local environment with pathological, enlarged lipid rafts (inflammarafts) [196][198] in membranes could exert a decisive influence over the selection process (Figure 2).
3.5. Melatonin Maintains a High Cytosolic ATP:ADP Ratio through the Optimization of VDAC-CYB5R3 Redox Complexes in Lipid Rafts
Lipid rafts are phase-separated regions in lipid bilayers responsible for important biological functions including signal transduction [92][93] as well as the trafficking and sorting of proteins and lipids [94][95]. The fact that lipid rafts are also important redox signaling platforms that assemble, recruit, and activate redox regulatory multiprotein complex NADPH oxidase [182][366], and host the quintessential plasma membrane redox enzyme complex VDAC-CYB5R3 [367][368], emphasizes the relevance of melatonin as an antioxidant in the protection and stabilization of lipid raft domains.
Nanoscopic transient lipid raft domains in biological membranes are formed by phase separation in response to external stimuli [92][93][188]. Even though cells may alter lipid constituents to control the composition and size of lipid rafts [315], the propagation of molecular stress, lipid raft rattling dynamics and relaxation are some of the basic mechanisms underlying phase separation on the molecular level [195]. The presence of hydrophobic molecules such as melatonin can modulate viscoelastic dynamics through the accumulation and propagation of stress in lipid–lipid interactions [195][316]. Adding melatonin to membrane models led to a breakdown of out-of-phase membrane displacement patterns and the disruption of the vibrational landing platform of lipid biomolecules at the water–membrane interface, effectively slowing the permeation of ROS and other small molecules [195][317].
In 2005, melatonin was first observed to induce phase-separation in DPPC lipid bilayers [318]; recently, melatonin has been observed to modify lipid hydrocarbon chain order to promote phase separation in ternary membrane models [319]. Due to a preference to localize at membrane interfaces [320], melatonin can form strong hydrogen bonds with membrane lipid anionic headgroups that could significantly modulate lipid acyl chain flexibility and lipid dynamics [318]. Melatonin is able to directly interact with cholesterol [321] and displaced cholesterol due to competitive binding to lipid molecules, increasing disorder in the Ld phase to drive cholesterol into the ordered Lo phase [319]. These subtle changes in lipid nanodomains can profoundly affect amyloid processing at membrane sites. Aβ1–40 and Aβ1–42 peptides are known to interact strongly with negatively charged lipids by binding to anionic, negatively charged membranes [322][323][324][325][326]. Increasing cholesterol content lowered the surface charge of lipid membranes in saline solution from positive to negative [327]. Although cholesterol is an indispensable constituent of lipid rafts [92][162], its electrostatic properties altered interactions of charged or polar biomolecules on lipid membrane surfaces and attracted the targeted binding of Aβ deposits at lipid membranes [328][329][330][331].
Local variations in melatonin concentration also affected the re-ordering of lipids in membranes. At 0.5 mol% concentration, melatonin was documented to penetrate lipid bilayers to form fluid domains that enriched lipid membranes where melatonin molecules aligned parallel to phospholipid tails with the electron-dense regions slightly below hydrophilic headgroups; however, at 30 mol% concentration, melatonin molecules aligned parallel to the lipid bilayer, close to the headgroup regions where one melatonin molecule was associated with two lipid molecules to form an ordered, uniform, lateral membrane structure distributed evenly throughout the membrane model [341]. Variations in local concentration and conformational changes in melatonin molecules can directly impact the lipid phase transition, line tension, size, health, and functions of lipid rafts.
Present in all eukaryotes [369], CYB5R3 encodes for a NADH-cytochrome b5 reductase 3 flavoprotein that is engaged in the one-electron transfer from NADH to cytochrome b5 or plasma membrane coenzyme Q, producing NAD+ as a result [370][371]. The soluble isoform of CYB5R3 is exclusive to erythrocytes [372], whereas the membrane-bound isoform is anchored to MOM, ER, and plasma membrane lipid rafts [368][373][374]. Importantly, the OMM-bound CYB5R3 enzyme, ubiquitously expressed in all mammalian cells, is functionally attached to the voltage-dependent anion channel 1 (VDAC1), one of the most prevalent proteins located in the OMM [375][376].
Originally known as mitochondrial porin after its identification in yeast (1985) [377] and humans (1989) [378], VDAC was subsequently observed as a resident protein of lipid rafts in the plasma membranes of animal hearts, brains, and lungs [379] from different human cell lines, including epithelial cells, astrocytes, and neurons [380][381]. Aberrant lipid composition in neuronal lipid rafts disturbs physiological VDAC protein interactions that can affect the opening and closing of VDAC channels, resulting in oxidative stress and neuronal impairments prominent in most AD pathologies [380]. The force-from-lipid principle dictates that the opening and closing of membrane embedded channels can be propelled by the mechanical properties of surrounding lipids [382][383][384][385] and their composition. Changes to raft thickness, curvature and elasticity [291] as a result of lipid peroxidation can therefore affect physiological functions of the VDAC and CYB5R3 redox complex.
CYB5R3 enzymes form large redox centers in lipid rafts that enhance mitochondrial respiration rate and ATP production, albeit resulting in increased production of ROS [368][373][374]. Over stimulation and clustering of CYB5R3 induced oxidative stress-mediated apoptosis of cerebellar granule neurons [386]. Independent of respiratory chain activities, the ascorbate-dependent NADH: cytochrome c oxidoreductase oxidation of NADH at CYB5R3 centers in lipid rafts is also a major source of extracellular superoxide [376][387][388][389][390] that can initiate lipid peroxidation. In Wistar rats, the deregulation of CYB5R3 promptly triggers apoptosis due to the overproduction of superoxide anions at neuronal plasma membranes [368][387]. Excess NADH due to CYB5R3 redox dysfunction can close VDAC, suppressing OXPHOS and increasing glycolysis [376][391], whereas the opening of VDAC also elevates ROS from increased OXPHOS activities [41]. As the most abundant protein in the MOM, VDAC is regarded as a dynamic regulator of mitochondrial functions, interacting with over 100 proteins in health and disease [392]. VDAC opening is believed to globally control mitochondrial metabolism and ROS formation, modulating mitochondria and cellular bioenergetics [41][393]. Nevertheless, the question of whether apoptosis is associated with the opening [394] or closure [395][396] of VDAC has been highly debated [397], further emphasizing the important role of this protein in the regulation of cell life and death [392][398].
VDAC is the gatekeeper which controls the export of ATP out of mitochondria into cytosol and the import of essential respiratory substrates such as ADP and Pi into mitochondria [395][399]; therefore, VDAC opening may be instrumental in determining the fate of MLO formation, regulation, and dissolution. ATP is not only a biological hydrotrope capable of inhibiting protein LLPS and aggregation at high mM concentrations, but it has recently been observed to act as a universal and specific regulator of IDRs capable of altering physicochemical properties, conformation dynamics, assembly, and the aggregation of MLOs [45]. Not only is the preservation of lipid raft structure and composition essential for maintaining specific ion channel properties [380], the amount of cytosolic ATP is dependent upon mitochondrial synthesis and the integrity of CL enriched raft-like lipid domains in mitochondria [367][400][401][402].
3.6. Melatonin May Regulate Glycolytic G Bodies by Increasing ATP
4. Conclusions
The physiological and pathological functions of biomolecular condensates in health and disease may be shaped by powerful, complex, interdependent relationships between membraneless organelles, membranes/lipid rafts, ATP, and most of all, stress and its timely resolution. Melatonin’s intimate association with each of these decisive influencers may position the potent, ancient antioxidant as an important mediator of the phase separation of condensates in health and disease via principal ATP-dependent post-translational mechanisms and regulation of ATP levels in mitochondria and cytoplasm (Figure 1). This novel theoretical review highlights the important connections between melatonin and ATP in the regulation of biomolecular condensates with the intention to spur further research interest and exploration in the full, multi-faceted potential of melatonin that may provide solutions and answers to existing and future challenges and questions in this exciting and promising field of study.
This entry is adapted from the peer-reviewed paper 10.3390/antiox10091483
