Pigments play a major role in many industries. Natural colors are usually much safer when compared to synthetic colors and may even possess some medicinal benefits. Synthetic colors are economical and can easily be produced compared to natural colors. In addition, raw plant materials for natural colors are limited and season dependent. Microorganisms provide an alternative source for natural colors and, among them, fungi provide a wide range of natural colorants that could easily be produced cheaply and with high yield. Along with pigment, some microbial strains are also capable of producing a number of mycotoxins. The commercial use of microbial pigments relies on the safety of colorants.
- fungal pigments
- mycotoxins
- safety evaluation
- pigment toxicity
1. Microbial Pigments
Natural pigments obtained from plants, animals and microorganisms are eco-friendly and have usually low or no toxicity [1][2]. The many disadvantages of using plants and animals prevent them from large-scale exploitation [3]. However, advantages of microbial pigments help to utilize their immense potential in various fields [4][5]. Even though the cost of microbial β-carotene production is several times more expensive, it can still compete with synthetic dyes in terms of it being natural and safe [6][7].
Microbial cells that produce color are referred to as microbial pigments producers [8]. They produce a wide range of colors (Figure 1) and are mostly water-soluble [9][10]. Natural pigments are mainly used as color additives or intensifiers; moreover, they are used as antioxidants and antibiotics (Figure 2). Due to indiscriminate use of synthetic colors and contrary reports on the safety of synthetic dyes, there is an important need to identify safe colorants from natural pigments. Microbial pigments have several advantages, viz., yield, cost efficiency, stability and ease of downstream processing compared to pigments from plant or animal origins [11][12].
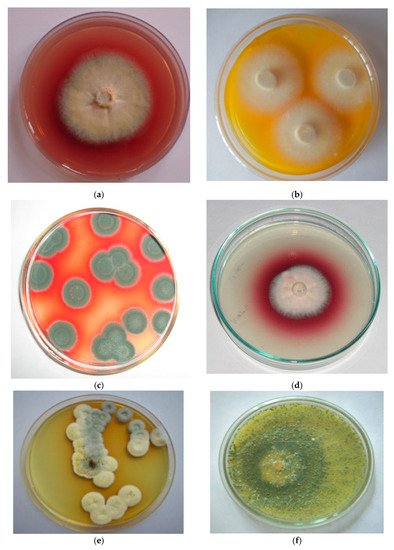
Figure 1. Pigments produced by different fungi: (a) Chaetomium sp. producing red pigment; (b) Thermomyces sp. producing yellow pigment; (c) Penicillium purpurogenum producing red pigment; (d) Fusarium sp. producing red pigment; (e) Penicillium purpurescens producing brown pigment (f) Trichoderma sp. producing yellow pigment.
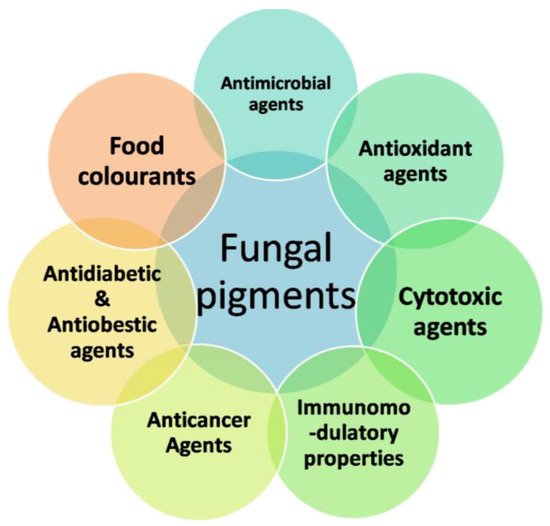
Figure 2. Applications of fungal pigments in the food industry.
Among pigment-producing microbes, fungi produce a wide range of water-soluble bio-pigments that have a variety of functions. Pigments extracted from fungi that are isolated from soil have various industrial applications. Filamentous fungi, viz., Monascus, Aspergillus, Penicillium, Neurospora, Eurotium, Drechslera and Trichoderma [13][14][15] are potential producers of bio-pigments. The pigments include carotenoids, melanins, flavins, phenazines, quinones, monacins and indigo [16]. Hence, they are the subject of many studies.
Recently, fungal pigments have been used for textile dyes, food colorants, antimicrobial and anticancer applications. They are also natural without having undesirable effects on the environment. Many scientific researchers have proved that pigments from soil fungi are a safer alternative to synthetic colorants, and there is good scope for industrial application [17][18].
2. Fungal Pigments and Toxicity Evaluation
Most fungi produce pigments along with mycotoxins. The presence of mycotoxins in pigments restricts the application of pigments as an additive in the food industry [1][19]. The European Union and the United States prohibit the consumption of Monascus pigments that are produced along with citrinin toxin, which poses a challenge over its safe use [20]. In short, natural pigments are a potential source of colorants that are eco-friendly, biodegradable, antimicrobial and have antioxidant properties. Apart from food additives, they are also used in cosmetics, pharmaceuticals and drug applications [21] (Table 1).
Table 1. Important fungal pigments and their safety evaluation.
| Fungus | Pigment(s) | Color | Mycotoxin(s) | Safety Evaluation | Biological Activity | Reference(s) |
|---|---|---|---|---|---|---|
| Aspergillus carbonarius | Melanins | Yellow | Not described/not found up to now | Subacute toxicity study | Antioxidant | [22] |
| Blackeslea trispora | ß-carotene | Red-orange | Aflatoxin Mycotoxin |
Genotoxicity and subacute toxicity study | Antioxidant, anticancer, suppression of cholesterol synthesis |
[23][24] |
| Fusarium graminearum | Rubrofusarin | Red | Fumonisins, Zearalenone, Fusaric Acid, Fusarins and Beauvericins | Cytotoxic in colon cells | Antimicrobial, antiallergic, phytotoxicic | [25][26][27] |
| Fusarium fujikuroi | Fusarubin | Orange | Fumonisins, Zearalenone, Fusaric Acid, Fusarins and Beauvericins | Cytotoxic against leukemia cells | Anticancer, antimicrobial | [27][28] |
| Fusarium oxysporum | Bikaverin | Red | Fumonisins, Zearalenone, Fusaric Acid, Fusarins and Beauvericins | Cytotoxic against tumour cells, apoptosis suppressor | Antimicrobial, antitumour | [29] |
| Monascus purpureus | Monascorubramine rubropunctamine | Red | Citrinin | Acute oral toxicity | Antihypertensive metabolite | [30] |
| Monascus anka | Ankaflavin and Monascin | Yellow | No coproduction of toxin | Acute oral toxicity | Antibacterial, antitumor and immunosuppressive | [31] |
| Monascus ruber | Monascorubrin and rubropunctatin | Orange-red | No coproduction of toxin | Oral toxicity | Anti-inflammatory, anticancer andantihyperlipidemic activities | [32] |
| Penicillium purpurogenum | Azaphilone | Brick red pigment | No coproduction of toxin | Brine shrimp Artemia salina study | Pharmaceutical and food industry | [33][34][35] |
| Purpurogenone | Yellow-orange | |||||
| Mitorubrino | Orange-red | |||||
| Mitorubrin | Yellow | |||||
| Penicillium europium | Benzoquinone | Pinkish red | Nontoxic | Subacute toxicity study | Antimicrobial | [36] |
| Penicillium resticulosum | Not described/not found up to now | Red | Nontoxic | Subacute toxicity study | Antimicrobial | [37] |
| Penicillium aculeatum | Ankaflavin | Yellow | Nontoxic, selective toxicity in cancer cells | Cytotoxicity study | Antimicrobial | [38] |
| Talaromycespurpureogenus | Purpuride, monascorubrin, purpurquinone-A, ankaflavin, | Yellow and red | Nontoxic | Subacute toxicity study | Antioxidant | [39] |
| Thermomyces sp. | Napthoquinone | Yellow | Nontoxic | Subacute toxicity study | Antioxidant, antimicrobial and food industry |
[40] |
| Trichoderma viride | Emodin Viridol |
Brown Yellow |
Nonphytotoxic | Phytotoxicity assay | Antimicrobial | [13] |
| Scytalidium cuboideum | Xylindein | Red | Nontoxic | Zebrafish toxicity study | UV resistant | [41] |
| Rhodotorula glutinis | β-carotene, torulene and torularhodin | Red and orange | Nontoxic | Standard subchronic toxicity study | Antioxidant, Antimicrobial Food and feed additive |
[42] |
| Rhodotorula gracilis | β-carotene, torulene and torularhodin | Red and orange | Nontoxic | Standard subchronic toxicity study | Antimicrobial | [43][44] |
| Yarrowia lipolytica | β-carotene | Brown | Nontoxic | Genotoxicity models and a standard subchronic toxicity study | Antimicrobial | [45] |
2.1. Aspergillus carbonarius
Aspergillus carbonarius, an Ascomycota fungus of the family Aspergillaceae, is capable of producing a yellow-colored pigment in its biomass. It does not produce any antinutrients or mycotoxins [46]. It has been exploited for large-scale production of polygalacturonase and is capable of temperature tolerance by UV irradiation when grown in shake-flask cultures. During the growth phase, a yellow colored pigment is accumulated in its biomass and has the potential to be used as a food colorant [47].
Toxicity studies in both sexes of albino rats at acute and subacute doses of the pigment revealed that feeding of fungal biomass did not show any mortality in rats and there are no significant differences in food intake or organ and body weight. When comparing treated and untreated rats, hematological parameters, serum enzymes lactate dehydrogenase (LDH), alkaline phosphatase (ALP), alanine aminotransferase (ALT or ALAT) and cholesterol assay also remain normal [48].
2.2. Blackslea trispora
Blakesleatrispora is a Zygomycetes fungus of the order Mucorales, family Choanephoraceae. It is capable of undergoing both sexual and asexual reproduction through the production of zygospores and sporangiospores. The fungus does not produce any toxic compounds; hence, it is of industrial interest as a source of β-carotene for commercial exploitation [49][50]. β-carotene from B. trispora was the first authorized microbial food colorant in the European Union. It is efficient and can achieve the highest yield of all trans β-carotene at the expense of other structurally related carotenoids [18]. The process production was improved over a number of years, producing carotenoid contents of up to 20% dry weight [51][52].
The safety assessment of β-carotene, derived from B. trispora, has revealed no genotoxicity or subacute toxicity for 4weeks [23][53]. A subchronic toxicity study of 90 days was performed with oral administration of F344.Rats of both sexes showed no adverse effects on their biological systems [24]. β-carotene derived from the B. trispora at a 5.0% dietary level, equivalent to 3127 mg/kg/day and 3362 mg/kg/day for male and female rats, caused no adverse effects. The findings revealed that the daily intake of synthetic β-carotene from B. trispora by human beings is a negligible toxicological hazard [54].
2.3. Fusarium sp.
Fusarium are Ascomycota fungi that belong to the order Hypocreales, family Nectriaceae. They produce a wide range of fungal pigments that are structurally and functionally diverse. However, among the Fusarium sp., Fusarium graminearum (red naphthoquinone pigment, rubrofusarin) [25], Fusarium fujikuroi (orange carotenoids pigment, fusarubin) [28][55] and Fusarium oxysporum (red naphthoquinone pigment, bikaverin) [29] are the major pigment-producing fungi. Secondary metabolites from these fungi contain numerous toxic compounds, viz., fumonisins, zearalenone, fusaric acid, fusarins and beauvericins [56].
Toxicity analysis revealed that the red dimeric naphthoquinone pigment from F. oxysporum-contaminated products affects human health. Recently, red naphthoquinone pigment has often been reported as a mycotoxin. However, naphthoquinone pigment was not genotoxic according to a DNA synthesis assay. Biotechnological approaches and intelligent screening of the toxic metabolite pathway of the pigment from Fusarium sp. will be helpful in producing the pigment for food coloring [26][27].
2.4. Monascus sp.
Monascus sp. are fungi placed under order Eurotiales, family Monascaceae. There are many species in this genus, among which M. purpureus (monascorubramine and rubropunctamine) [30], M. anka (ankaflavin and monascin) [31] and M. ruber (monascorubrinandrubropunctatin) [32] are of greatest significance to the food industry. Traditionally, Monascus pigments were produced on rice using solid-state microbial fermentation. Synonyms for this food product include, Hon-Chi, Hong Qu, Dan Qu, Anka, Ankak rice, Beni-Koji, red koji, red Chinese rice, red yeast rice and red mold rice (RMR). RMR was utilized as a food colorant in traditional Chinese medicine for more than 1000 years [57].
Chinese, as well as other East Asian people, have confirmed the safety of red yeast rice. The European Food Safety Authority (EFSA) and the United States excluded red yeast rice on the list of permissible food additives, due to complex secondary metabolites [58][59]. The toxigenic strain of Monascus purpureus is capable of producing nephrotoxic and hepatotoxic mycotoxin citrinin, which limits the wide application of the pigment [60].
For more than a thousand years, pigments produced by Monascus sp. were legally used as food colorants in South East Asia, even though they were demonstrated to have physiological effects. There are numerous toxicological data available on this Monascus red pigment.
A genetically modified industrial strain, M. purpureus SM001 isolated in China, is capable of producing pigment without citrinin, which is the best Monascus pigment producer.This results in the prolonged safety of Monascus-related products and their application [61].
2.5. Penicillium sp.
Penicillium are Ascomycota fungi belonging to the order Eurotiales, family Trichocomaceae. They are capable of producing many pigments. Penicillium are ubiquitous saprophytic soil fungi, present wherever organic material is available. Several species are capable of producing highly toxic mycotoxins. Some species of the genus Penicillium are capable of producing antibiotics, while some other species are used in cheese making; however, pigment production by these fungi is less well known [34][62]. Patents contain information about acute oral toxicity in mice. A 90-day subchronic toxicological study found acute dermal irritation, acute eye irritation, antitumor activity, micronucleus test in mice, AMES test (Salmonella typhimurium reverse mutation assay) and an estimation of antibiotic activity, including results of estimation of five mycotoxins [63].
Penicillium purpurogenum is capable of producing an azaphilone-like pigment. It secretes a brick red pigment during growth, which generally diffuses into commonly used media. However, violet pigment (PP-V) and orange pigment (PP-O) were also reported by altering culture conditions [64]. The production of pigment from Penicillium is more efficient and profitable than any other microorganism. It secretes enzymes and pigments out of the cell and the secreted pigment is water-soluble and relatively stable; thus, it is easily purified [65].
Toxicity studies of P. purpurogenum DPUA 1275 on brine shrimp, Artemia salina, showed antimicrobial effects and absence of toxicity to go along with pigment production. It also does not produce any known mycotoxins and is nonpathogenic to humans. It is a potential strain for the production of food pigments [66]. Although many species of Penicillium are found to produce pigments, only a few toxicological studies have been conducted.
Penicillium europium, isolated from forest soil, is capable of producing a pinkish pigment by using longifolene as a sole carbon source. A toxicity study on albino rats revealed that the pigment had no toxic effect on rats. Synthesized pigments from P. europium could be used in food, feed and pharmaceutical industries. Apart from the food industry, it could be used for various industrial applications, viz., dyes for textile and non-textile substrates such as paper, leather, paints and cosmetics. Moreover, as it is non cytotoxic, the pigment could be a potential replacement for hazardous synthetic dyes [36][67].
Penicillium resticulosum is capable of producing red pigments. An evaluation of the subacute toxicity of oral exposure on the synthesized pigment on adult male and female mice for 28 days, using a pigment dose of up to 500 mg kg−1 body weight daily, had no effect on body weight, organ weight, or the activity of lactate dehydrogenase (LDH), alkaline phosphatase (ALP), alanine aminotransferase (ALT or ALAT) enzymes or blood urea nitrogen (BUN) levels. However, mice taking the pigment over 500 mg·kg−1 body weight daily showed fatty degeneration and mild necrosis of liver cells, indicating that doses under 500 mg·kg−1 body weight were safe for daily consumption [37][68].
Penicillium aculeatum produces a yellow (ankaflavin) pigment under submerged fermentation. Cytotoxicity studies of the pigment interacting with human colon carcinoma cell lines (HCT116) and human prostatic carcinoma cell lines (PC3) exhibited apoptosis and cell cycle inhibition at lower concentrations. An assay of human erythrocytes and human embryonic kidney (HEK-293) cell lines showed the least cytotoxicity atfor highest concentrations tested. Displaying selective cytotoxicity is an important property for an ideal anticancer drug [38].
This entry is adapted from the peer-reviewed paper 10.3390/jof7090692
References
- Dufossé, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61.
- Gupta, N.; Poddar, K.; Sarkar, D.; Kumari, N.; Padhan, B.; Sarkar, A. Fruit waste management by pigment production and utilization of residual as bioadsorbent. J. Environ. Manag. 2019, 244, 138–143.
- Aruldass, C.A.; Dufossé, L.; Ahmad, W.A. Current perspective of yellowish-orange pigments from microorganisms—A review. J. Clean. Prod. 2018, 180, 168–182.
- Panesar, R.; Kaur, S.; Panesar, P. Production of microbial pigments utilizing agro-industrial waste: A review. Curr. Opin. Food Sci. 2015, 1, 70–76.
- Tirumale, S.; Wani, N.A. Biopigments: Fungal Pigments; Springer: Singapore, 2018.
- Nigam, P.S.; Luke, J.S. Food additives: Production of microbial pigments and their antioxidant properties. Curr. Opin. Food Sci. 2016, 7, 93–100.
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial pigments and their applications. Process. Biochem. 2013, 48, 1065–1079.
- Joshi, V.A.; Bala, A.; Bhushan, S. Microbial Pigments. Indian J. Biotechnol. 2003, 2, 362–369.
- Chattopadhyay, P.; Chatterjee, S.; Sen, S.K. Biotechnological potential of nature food grade biocolourants. Afr. J. Biotechnol. 2008, 7, 2972–2985.
- Lin, L.; Xu, J. Fungal Pigments and Their Roles Associated with Human Health. J. Fungi 2020, 6, 280.
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial pigments as natural color sources: Current trends and future perspectives. J. Food Sci. Technol. 2015, 52, 4669–4678.
- Velmurugan, P.; Kim, M.-J.; Park, J.-S.; Karthikeyan, K.; Lakshmanaperumalsamy, P.; Lee, K.-J.; Park, Y.-J.; Oh, B.-T. Dyeing of cotton yarn with five water soluble fungal pigments obtained from five fungi. Fibers Polym. 2010, 11, 598–605.
- Nirlane da Costa Souza, P.; Luiza Bim Grigoletto, T.; Alberto Beraldo de Moraes, L.; Abreu, L.M.; Henrique Souza Guimarães, L.; Santos, C.; Ribeiro Galvão, L.; Gomes Cardoso, P. Production and chemical characterization of pigments in filamentous fungi. Microbiology 2016, 162, 12–22.
- Heo, Y.M.; Kim, K.; Kwon, S.; Na, J.; Lee, H.; Jang, S.; Kim, C.-H.; Jung, J.; Kim, J.-J. Investigation of Filamentous Fungi Producing Safe, Functional Water-Soluble Pigments. Mycobiology 2018, 46, 269–277.
- Mostafa, M.; Abbady, M. Secondary Metabolites and Bioactivity of the Monascus Pigments—Review Article. Glob. J. Biotechnol. Biochem. 2014, 9, 1–13.
- Saravanan, A.; Jayasree, R.; Kumar, P.S.; Varjani, S.; Hemavathy, R.; Jeevanantham, S.; Yaashikaa, P.R. Production of pigment using Aspergillus tamarii: New potentials for synthesizing natural metabolites. Environ. Technol. Innov. 2020, 19, 100967.
- Kongsak Boonyapranai, R.T. Sorasak Lhieochaiphant and Suree Phutrakul. Optimization of Submerged Culture for the Production of Naphthoquinones Pigment by Fusarium verticillioides. Chiang Mai J. Sci. 2008, 35, 457–466.
- Dufossé, L. Microbial Production of Food Grade Pigments. Food Technol. Biotechnol. 2006, 44, 313–321.
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal Pigments and Their Prospects in Different Industries. Microorganisms 2019, 7, 604.
- Carvalho, J.C.; Oishi, B.O.; Pandey, A.; Soccol, C. Biopigments from Monascus: Strains selection, citrinin production and color stability. Braz. Arch. Biol. Technol. 2005, 48, 885–894.
- Dufossé, L. 16—Current and Potential Natural Pigments From Microorganisms (Bacteria, Yeasts, Fungi, Microalgae). In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 337–354.
- Bezirhan Arikan, E.; Canli, O.; Caro, Y.; Dufossé, L.; Dizge, N. Production of Bio-Based Pigments from Food Processing Industry By-Products (Apple, Pomegranate, Black Carrot, Red Beet Pulps) Using Aspergillus carbonarius. J. Fungi 2020, 6, 240.
- The Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants. FAO/WHO Expert Committee on Food Additive; World Health Organization: Geneva, Switzerland, 1996; pp. 1–100.
- Woutersen, R.A.; Wolterbeek, A.P.; Appel, M.J.; van den Berg, H.; Goldbohm, R.A.; Feron, V.J. Safety evaluation of synthetic beta-carotene. Crit. Rev. Toxicol. 1999, 29, 515–542.
- Cambaza, E. Comprehensive Description of Fusarium graminearum Pigments and Related Compounds. Foods 2018, 7, 165.
- Díaz-Sánchez, V.; Avalos, J.; Limón, M.C. Identification and regulation of fusA, the polyketide synthase gene responsible for fusarin production in Fusarium fujikuroi. Appl. Environ. Microbiol. 2012, 78, 7258–7266.
- Soumya, K.; Narasimha Murthy, K.; Sreelatha, G.L.; Tirumale, S. Characterization of a red pigment from Fusarium chlamydosporum exhibiting selective cytotoxicity against human breast cancer MCF-7 cell lines. J. Appl. Microbiol. 2018, 125, 148–158.
- Menezes, B.S.; Solidade, L.S.; Conceição, A.A.; Santos Junior, M.N.; Leal, P.L.; de Brito, E.S.; Canuto, K.M.; Mendonça, S.; de Siqueira, F.G.; Marques, L.M. Pigment production by Fusarium solani BRM054066 and determination of antioxidant and anti-inflammatory properties. AMB Express 2020, 10, 117.
- Santos, M.C.d.; Mendonça, M.d.L.; Bicas, J.L. Modeling bikaverin production by Fusarium oxysporum CCT7620 in shake flask cultures. Bioresour. Bioprocess. 2020, 7, 13.
- Mohankumari, H.P.; Naidu, K.A.; Narasimhamurthy, K.; Vijayalakshmi, G. Bioactive Pigments of Monascus purpureus Attributed to Antioxidant, HMG-CoA Reductase Inhibition and Anti-atherogenic Functions. Front. Sustain. Food Syst. 2021, 5, 590427.
- Shi, K.; Tang, R.; Huang, T.; Wang, L.; Wu, Z. Pigment fingerprint profile during extractive fermentation with Monascus anka GIM 3.592. BMC Biotechnol. 2017, 17, 46.
- Darwesh, O.M.; Matter, I.A.; Almoallim, H.S.; Alharbi, S.A.; Oh, Y.-K. Isolation and Optimization of Monascus ruber OMNRC45 for Red Pigment Production and Evaluation of the Pigment as a Food Colorant. Appl. Sci. 2020, 10, 8867.
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15.
- Mapari, S.A.S.; Meyer, A.S.; Thrane, U.; Frisvad, J.C. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb. Cell Factories 2009, 8, 24.
- Mapari, S.A.S.; Meyer, A.S.; Thrane, U.; Frisvad, J. Production of Monascus-like Pigments. European Patent EP2262862B1, 28 March 2012.
- Khan, A.A.; Iqubal, S.S.; Shaikh, I.A.; Niyongabo Niyonzima, F.; More, V.S.; Muddapur, U.M.; Bennur, R.S.; More, S.S. Biotransformation of longifolene by Penicillium europium. Biocatal. Biotransform. 2021, 39, 41–47.
- Sopandi, T.; Wardah, W. Sub-Acute Toxicity of Pigment Derived from Penicillium resticulosum in Mice. Microbiol. Indones. 2012, 6, 6.
- Krishnamurthy, S.; Narasimha Murthy, K.; Thirumale, S. Characterization of ankaflavin from Penicillium aculeatum and its cytotoxic properties. Nat. Prod. Res. 2020, 34, 1630–1635.
- Gopal Pandit, S.; Honganoor Puttananjaiah, M.; Serva Peddha, M.; Appasaheb Dhale, M. Corrigendum to ‘Safety efficacy and chemical profiling of water-soluble Talaromyces purpureogenus CFRM02 pigment’ [Food Chem. 310 (2020) 125869]. Food Chem. 2020, 317, 126403.
- Poorniammal, R.; Gunasekaran, S.; Sriharasivakumar, H. Toxicity evaluation of funfal food colourant from Thermomyces sp in albino mice. J. Sci. Ind. Res. 2011, 70, 773–777.
- Almurshidi, B.H.; Van Court, R.C.; Vega Gutierrez, S.M.; Harper, S.; Harper, B.; Robinson, S.C. Preliminary Examination of the Toxicity of Spalting Fungal Pigments: A Comparison between Extraction Methods. J. Fungi 2021, 7, 155.
- Latha, B.V.; Jeevaratanm, K. Thirteen-week oral toxicity study of carotenoid pigment from Rhodotorula glutinis DFR-PDY in rats. Indian J. Exp. Biol. 2012, 50, 645–651.
- Naidu, K.A.; Venkateswaran, G.; Vijayalakshmi, G.; Manjula, K.; Viswanatha, S.; Murthy, K.N.; Srinivas, L.; Joseph, R. Toxicological assessment of the yeast Rhodotorula gracilis in experimental animals. Z. Für Lebensm. Und Forsch. A 1999, 208, 444–448.
- Kot, A.M.; Błażejak, S.; Gientka, I.; Kieliszek, M.; Bryś, J. Torulene and torularhodin: “new” fungal carotenoids for industry? Microb. Cell Fact. 2018, 17, 49.
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206.
- Venkatesh, K.S. Strain and Process Improvement for Polygalacturonase Production by Aspergillus carbonarius. Ph.D. Thesis, University of Mysore, Mysore, India, 2004.
- Narendrababu, B.N.; Shishupala, S. Spectrophotometric detection of pigments from Aspergillus and Penicillium isolates. J. Appl. Biol. Biotechnol. 2017, 5, 53–58.
- Sanjay, K.R.; Kumaresan, N.; Akhilender Naidu, K.; Viswanatha, S.; Narasimhamurthy, K.; Umesh Kumar, S.; Vijayalakshmi, G. Safety evaluation of pigment containing Aspergillus carbonarius biomass in albino rats. Food Chem. Toxicol. 2007, 45, 431–439.
- Mantzouridou, F.; Tsimidou, M.Z. On the monitoring of carotenogenesis by Blakeslea trispora using HPLC. Food Chem. 2007, 104, 439–444.
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial Pigments in the Food Industry-Challenges and the Way Forward. Front. Nutr. 2019, 6, 7.
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M. Substrate contribution on carotenoids production in Blakeslea trispora cultivations. Food Bioprod. Process. 2010, 88, 305–311.
- Papadaki, E.; Mantzouridou, F. Natural ²-Carotene Production by Blakeslea trispora Cultivated in Spanish-Style Green Olive Processing Wastewaters. Foods 2021, 10, 327.
- Finkelstein, M.; Huang, C.C.; Byng, G.S.; Tsau, B.R.; Leach, J. Blakeslea Trispora Mated Culture Capable of Increased Beta-carotene Production. U.S. Patent US5422247A, 6 June 1995.
- Nabae, K.; Ichihara, T.; Hagiwara, A.; Hirota, T.; Toda, Y.; Tamano, S.; Nishino, M.; Ogasawara, T.; Sasaki, Y.; Nakamura, M.; et al. A 90-day oral toxicity study of beta-carotene derived from Blakeslea trispora, a natural food colorant, in F344 rats. Food Chem. Toxicol. 2005, 43, 1127–1133.
- Tudzynski, B. Gibberellin biosynthesis in fungi: Genes, enzymes, evolution, and impact on biotechnology. Appl. Microbiol. Biotechnol. 2005, 66, 597–611.
- Avalos, J.; Prado-Cabrero, A.; Estrada, A.F. Neurosporaxanthin production by Neurospora and Fusarium. Methods Mol. Biol. 2012, 898, 263–274.
- Ma, J.; Li, Y.; Ye, Q.; Li, J.; Hua, Y.; Ju, D.; Zhang, D.; Cooper, R.; Chang, M. Constituents of red yeast rice, a traditional Chinese food and medicine. J. Agric. Food Chem. 2000, 48, 5220–5225.
- Chen, W.; He, Y.; Zhou, Y.; Shao, Y.; Feng, Y.; Li, M.; Chen, F. Edible Filamentous Fungi from the Species Monascus: Early Traditional Fermentations, Modern Molecular Biology, and Future Genomics. Compr. Rev. Food Sci. Food Saf. 2015, 14, 555–567.
- Kallscheuer, N. Engineered Microorganisms for the Production of Food Additives Approved by the European Union—A Systematic Analysis. Front. Microbiol. 2018, 9, 1746.
- Liu, J.; Zhou, Y.; Yi, T.; Zhao, M.; Xie, N.; Lei, M.; Liu, Q.; Shao, Y.; Chen, F. Identification and role analysis of an intermediate produced by a polygenic mutant of Monascus pigments cluster in Monascus ruber M7. Appl. Microbiol. Biotechnol. 2016, 100, 7037–7049.
- Blanc, P.J.; Loret, M.O.; Goma, G. Production of citrinin by various species ofMonascus. Biotechnol. Lett. 1995, 17, 291–294.
- Gunasekaran Sanjeevi, P.R. Optimization of fermentation conditions for red pigment production from Penicillium sp. under submerged cultivation. Afr. J. Biotechnol. 2008, 7, 1894–1898.
- Sardaryan, E.; Zihlova, H.; Strnad, R.; Cermakova, Z. Arpink red—meet a new natural red food colourant of microbial origin. In Pigments in Food, More Than Colours; Elsevier: Amsterdam, TheNetherlands, 2004; pp. 207–208.
- Kojima, R.; Arai, T.; Matsufuji, H.; Kasumi, T.; Watanabe, T.; Ogihara, J. The relationship between the violet pigment PP-V production and intracellular ammonium level in Penicillium purpurogenum. AMB Express 2016, 6, 43.
- Corrêia Gomes, D.; Takahashi, J.A. Sequential fungal fermentation-biotransformation process to produce a red pigment from sclerotiorin. Food Chem. 2016, 210, 355–361.
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371.
- Khan, A.A.; Alshabi, A.M.; Alqahtani, Y.S.; Alqahtani, A.M.; Bennur, R.S.; Shaikh, I.A.; Muddapur, U.M.; Iqubal, S.M.S.; Mohammed, T.; Dawoud, A.; et al. Extraction and identification of fungal pigment from Penicillium europium using different spectral studies. J. King Saud Univ. Sci. 2021, 33, 101437.
- Sethi, B.K.P.; Parida, P.; Sahoo, S.L.; Dikshit, B.; Pradhan, C.; Sena, S.; Behera, B.C. Extracellular production and characterization of red pigment from Penicillium purpurogenum BKS9. Alger. J. Nat. Prod. 2016, 4, 379–392.
