Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
Phenolic acids comprise a class of phytochemical compounds that can be extracted from various plant sources and are well known for their antioxidant and anti-inflammatory properties.
- phenolic acids
- antioxidants
- health properties
1. Introduction
Free radicals are mainly reactive oxygen species (ROS) (including hydroxyl-, superoxide-radicals and singlet oxygen) that are formed in tissue cells by various endogenous and exogenous pathways. ROS normally exert an adverse impact on human health by inducing the so called “oxidative stress conditions” [1]. The ability of free radicals to structurally modify cellular components and cause oxidative damage to biomolecules (LDL-low density lipoproteins, DNA, etc.) has revealed their involvement in a variety of health pathologies (i.e., inflammation, aging, types of cancer and cardiovascular diseases) [2][3].
Nature has generously offered several types of natural dietary antioxidants, among which phenolic compounds can operate as scavengers of free radicals in vivo and can efficiently reduce the harmful health impacts of oxidative damage [4][5]. Phenolic acids comprise a group of natural phenolic compounds that are present in a wide range of herbs and other species of the plant kingdom [6]. More specifically, thyme, oregano, rosemary, sage, and mint herbal preparations—all rich in various phenolics—have been reported to exert strong antioxidant biochemical and anti-inflammatory properties [7][8]. A few authors have reviewed the radical scavenging capacity of phenolic acids and their subsequent beneficial effects against the development of cancer, cardiovascular diseases and other health disorders (such as skin problems, inflammations, bacterial infections, etc.) [9]. The main biochemical pathways and mechanisms of phenolic actions against the development of certain types of cancer include: free radical scavenging, enzyme induction, DNA damage repair, cell proliferation depression, and apoptosis [10].
2. Structural Classification of Natural Phenolic Acids
In terms of their chemical structure, phenolic acids are classified as:
Hydroxybenzoic acids with a C6-C1 structure: Among them a trihydroxy derivative (gallic acid) has been associated with tea antioxidant activity, while vanilic acid is a methoxy-hydroxy derivative serving as a well-known flavouring agent [11].
Hydroxycinnamic acids with a C6-C3 structure[12]: These are abundant in plant sources, with p-coumaric (4-hydroxy derivative), caffeic (3, 4-dihydroxy derivative) and ferulic (3-methoxy, 4-hydroxy derivative) commonly present in various culinary herbs. In addition, rosmarinic acid (an ester of caffeic acid with 3,4-dihydroxyphenyl lactic acid) is mainly encountered in certain aromatic herbs [13].
Phenylacetic acids with a C6-C2 structure. Phenylacetic acids are scarce in fruits and vegetables, while a dihydroxy derivative was detected in strawberry tree honey [14]. Carnosic acid belongs to the phenolic diterpenes that are usually classified as hybrid phenolics [12].
This review focuses on the most common hydroxybenzoic and hydroxycinnamic phenolic acids, along with carnosic acid, the chemical structures of which are given in Figure 1.
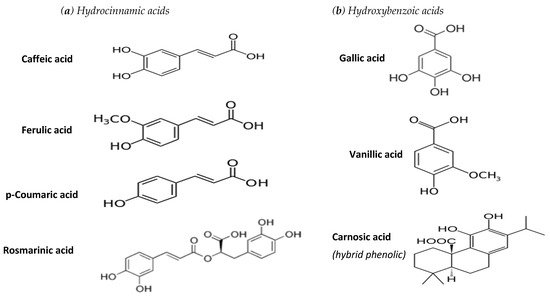
Figure 1. Chemical structure of phenolic acids examined in this study.
3. Herbal Sources and Extraction of Phenolic Acids
Caffeic acid (CA) is found at high levels in various herbs worldwide, including the South American herb yerba mate (1.5 g/kg) [15], the Japanese herbal leaf tea, the tea stem from Moringa oleifera L. [16], and thyme (1.7 mg/kg) [17].
Carnosic acid (CarA) can be found in a few species of the Lamiaceae family (such as rosemary and common salvia species). It has been reported to be present at a concentration of 1.5 to 2.5% in dried sage leaves [18][19].
Ferulic acid (FA) is present in black beans at an average concentration of 0.8 g/kg, while flaxseed has been reported as the richest natural source of FA glucoside (4.1 ± 0.2 g/kg), [20][21]. FA has been also identified as the major phenolic acid in Angelica sinensis (Oliv.), a traditional medicinal and edible plant in China [22].
Gallic acid (GA) has been found to be the main phenolic acid in tea [23] but also reported in high amounts in the parasitic plant Cynomorium coccineum, the aquatic plant Myriophyllum spicatum, and the blue-green alga Microcystis aeruginosa [24]. In addition, GA was recently identified as the main phenolic compound in leaf extracts from the medicinal halophyte Thespesia populnea tea [25].
p-Coumaric acid (p-CA) has been identified in basil, garlic [26] and in amaranth leaves and stem at a concentration range of 28–44 mg/kg [27]. p-CA has been reported as the major active compound in Bambusae Caulis, a Chinese medicinal herb [28] as well as in cultivars of husked oat (Avena sativa L.) in Finland [29].
Rosmarinic acid (RA) is the main phenolic component in several members of the Lamiaceae family, including among others: Rosmarinus officinalis, Origanum spp., Perilla spp., and Salvia officinialis in concentrations varying between 0.05 and 26 g/kg dry weight [30]. Additionally, the results of Tsimogiannis et al. [31] indicate an amount of 19.5 g/kg in the leaves of pink savoury (Satureja thymbra L.).
Vanillic Acid (VA) is commonly found in several fruits, olives, and cereal grains (e.g., whole wheat), as well as in wine. VA was also identified in fruit extracts of the açaí palm plant (Euterpe oleracea) [32] and in the root of Angelica sinensis (an herb indigenous to China) at concentrations between 1.1 and 1.3 g/kg [33].
4. Extraction of Phenolic Acids from Their Natural Sources
The extraction and identification of phenolic acids has been studied by various researchers [34][35]. Phenolic acids are compounds with medium to high polarity and, therefore, can be extracted by water [36]. Nevertheless, aqueous solutions of ethanol or acetone (50–70%) are the best solvents for the quantitative extraction of hydrocinnamic acids [37]. On the contrary, CarA exhibits low polarity and is quantitatively extracted with the use of pure acetone or ethanol [38].
Hydroxycinnamic and hydroxybenzoic acids may be linked to polysaccharides of the cell walls by ester bonds and to lignin components by ester or ether bonds [39]. Mild alkaline hydrolysis can be implied to cleave the ester bonds, while acid hydrolysis to cleave the ether bonds and release the phenolic acids [40]. However, phenolic acids may be degraded under alkaline conditions, e.g., RA has been reported to transform to CA [41]. Additionally, mild temperature and time combinations are suggested to avoid degradation. The most prone to degradation is CarA, which is oxidised to carnosol (which also exhibits antioxidant activity) at temperatures higher than 50 °C and at longer extraction times [42].
In addition to conventional solid liquid extraction, ultrasound assisted extraction and microwave assisted extraction proved even more effective for phenolic acid extraction, while shortening extraction time [43][44].
The predominant role of high-performance liquid chromatography (HPLC) in the definition of the phenolic profile of various plant sources has been recently examined by Ciulu et al. (2018) [45], who also present the most recently developed mass spectrometry-based detection systems. In addition, the various developed procedures for the quantification of phenolic compounds have been described in the literature, along with the spectrophotometric protocols for the evaluation of their antioxidant properties [46][47].
This entry is adapted from the peer-reviewed paper 10.3390/molecules26175405
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogen. Rev. 2010, 4, 118–126.
- Yan, M.; Lo, C.-J.; Edwards, T.-J.; Baran, S.-P. Radicals: Reactive intermediates with translational potential. J. Am. Chem. Soc. 2016, 138, 12692–12714.
- Dizdaroglu, M.; Jaruga, P. Mechanisms of free radical-induced damage to DNA. Free Radic. Res. 2012, 46, 382–419.
- Mamede, A.C.; Tavares, S.D.; Abrantes, A.M.; Trindade, J.; Maia, J.M.; Botelho, M.F. The role of vitamins in cancer: A review. Nutr. Cancer 2011, 63, 479–494.
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Effect of natural Food Antioxidants against LDL and DNA Oxidative damages. Antioxidants 2018, 7, 133.
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Beneficial Health Properties of Common Natural Phenolic Acids. Encyclopedia. 2020. Available online: https://encyclopedia.pub/1205 (accessed on 13 January 2021).
- Yemiş, G.P.; Pagotto, F.; Bach, S.; Delaquis, P. Effect of Vanillin, Ethyl Vanillin, and Vanillic Acid on the Growth and Heat Resistance of Cronobacter Species. J. Food Protect. 2011, 74, 2062–2069.
- Jungbauer, A.; Medjakovic, S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas 2012, 71, 227–239.
- Kiokias, S. Antioxidant effects of vitamins C, E and provitamin A compounds as monitored by use of biochemical oxidative indicators linked to atherosclerosis and carcinogenesis. Intern. J. Nutr. Res. 2019, 1, 1–13.
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115.
- Liu, Y.; Carver, J.A.; Calabrese, A.N.; Pukala, T.L. Gallic acid interacts with α-synuclein to prevent the structural collapse necessary for its aggregation. Biochim. Biophys. Acta (BBA)-Prot. Proteom. 2014, 1844, 1481–1485.
- Tsimogiannis, D.; Oreopoulou, V. Classification of phenolic compounds in Plants. In Polyphenols in Plants Isolation Purification and Extract Preparation, 2nd ed.; Watson, R.R., Ed.; Elsevier Inc.: London, UK, 2019; pp. 263–284.
- Dalbem, L.; Costa Monteiro, C.M.; Anderson, J.T. Anticancer properties of hydroxycinnamic acids—A Review. Canc. Clin. Oncol. 2012, 1, 109–121.
- Cabras, P.; Angioni, A.; Tuberoso, C.; Floris, I.; Reniero, F.; Guillou, C.; Ghelli, S. Homogentisic acid: A phenolic acid as a marker of strawberry-tree (Arbutus unedo) honey. J. Agric. Food Chem. 1999, 47, 4064–4067.
- Berté, K.A.; Beux, M.R.; Spada, P.K.; Salvador, M.; Hoffmann-Ribani, R. Chemical composition and antioxidant activity of yerba-mate (Ilex paraguariensis A.St.-Hil., Aquifoliaceae) extract as obtained by spray drying. J. Agric. Food Chem 2011, 25, 5523–5527.
- Sugahara, S.; Chiyo, A.; Fukuoka, Κ.; Ueda, Y.; Tokunaga, Y.; Nishida, Y.; Kinoshita, H.; Matsuda, Y.; Igoshi, K.; Ono, M.; et al. Unique antioxidant effects of herbal leaf tea and stem tea from Moringa oleifera L. especially on superoxide anion radical generation systems. Biosci. Biotechnol. Biochem. 2018, 82, 1973–1984.
- Žugić, A.; Đorđević, S.; Arsić, I.; Marković, G.; Živković, J.; Jovanovic, S.; Tadić, V. Antioxidant activity and phenolic compounds in 10 selected herbs from Vrujci Spa, Serbia. Indust. Crops Prod. 2014, 52, 519–527.
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic Acid and Carnosol, Two Major Antioxidants of Rosemary, Act through Different Mechanisms. Plant Physiol. 2017, 175, 1381–1394.
- Raes, K.; Doolaege, E.H.A.; Deman, S.; Vossen, E.; De Smet, S. Effect of carnosic acid, quercetin and α-tocopherol on lipid and protein oxidation in anin vitrosimulated gastric digestion model. Intern. J. Food Sci. Nutr. 2015, 66, 216–221.
- Flanagan, J.; Bily, A.; Rolland, Y.; Roller, M. Lipolytic Activity of Svetol®, a Decaffeinated Green Coffee Bean Extract. Phytoth. Res. 2013, 28, 946–948.
- Mojica, L.; Meyer, A.; Berhow, M.; González, E. Bean cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase while diverse phenolic composition and concentration. Food Res. Intern. 2015, 69, 38–48.
- Wei, W.L.; Zeng, R.; Gu, C.M.; Qu, Y.; Huang, L.F. Angelica sinensis in China-A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J. Ethnopharmacol. 2016, 190, 116–141.
- Pandurangan, A.K.; Mohebali, N.; Norhaizan, M.E.; Looi, C.Y. Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Des. Dev. Ther. 2015, 9, 3923–3934.
- Zucca, P.; Rosa, A.; Tuberoso, C.; Piras, A.; Rinaldi, A.C.; Sanjust, E.; Dessi, A.; Rescigno, A. Evaluation of Antioxidant Potential of “Maltese Mushroom” (Cynomorium coccineum) by Means of Multiple Chemical and Biological Assays. Nutrients 2013, 5, 149–161.
- Rangani, I.; Kumari, A.; Patel, M.; Brahmbhatt, H.; Parida, A.K. Phytochemical profiling, polyphenol composition, and antioxidant activity of the leaf extract from the medicinal halophyte Thespesia populnea reveal a potential source of bioactive compounds and nutraceuticals. J. Food Biochem. 2019, 43, e12731.
- Trisha, S. Role of hesperdin, luteolin and coumaric acid in arthritis management: A Review. Int. J. Phys. Nutr. Phys. Educ. 2018, 3, 1183–1186.
- Kavita, P.; Gandhi, P. Rediscovering the therapeutic potential of Amaranthus species: A review. Egypt J. Basic Appl. Sci. 2017, 4, 196–205.
- Woogyeong, K.; Dahae, L.; Jinju, K. p-Coumaric Acid, a Major Active Compound of Bambusae Caulis in Taeniam, Suppresses Cigarette Smoke-Induced Pulmonary Inflammation. Am. J. Chin. Med. 2018, 46, 407–421.
- Multari, S.; Pihlava, J.M.; Ollennu-Chuasam, P.; Hietaniemi, V.; Yang, B.; Suomela, J.P. Identification and Quantification of Avenanthramides and Free and Bound Phenolic Acids in Eight Cultivars of Husked Oat (Avena sativa L) from Finland. J. Agric. Food Chem. 2018, 68, 2900–2908.
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, V. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70.
- Tsimogiannis, D.; Choulitoudi, E.; Bimpilas, A.; Mitropoulou, G.; Kourkoutas, Y.; Oreopoulou, V. Exploitation of the biological potential of Satureja thymbra essential oil and distillation by-products. J. Appl. Res. Med. Aromat. Plants 2016, 4, 12–20.
- Pacheco-Palencia, L.A.; Mertens, T.S.; Talcott, S.-T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636.
- Chengke, Z.; Yuan, J.; Fachuang, L. Angelica Stem: A Potential Low-Cost Source of Bioactive Phthalides and Phytosterols. Molecules 2018, 23, 3065.
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols From Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants Isolation Purification and Extract Preparation, 2nd ed.; Watson, R.R., Ed.; Elsevier Inc.: London, UK, 2019; pp. 243–260.
- Psarrou, I.; Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction Kinetics of Phenolic Antioxidants from the Hydro Distillation Residues of Rosemary and Effect of Pretreatment and Extraction Parameters. Molecules 2020, 25, 4520.
- Oreopoulou, A.; Goussias, G.; Tsimogiannis, D.; Oreopoulou, V. Hydro-alcoholic Extraction Kinetics of Phenolics from Oregano: Optimization of the Extraction Parameters. Food Bioprod. Proc. 2020, 123, 378–389.
- Corbin, C.; Fidel, T.; Leclerc, E.A.; Barakzoy, E.; Sagot, N.; Falguiéres, A.; Lainé, E. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultras. Sonochem. 2015, 26, 176–185.
- De AR Oliveira, G.; De Oliveira, A.E.; Da Conceição, E.C.; Leles, M.I. Multiresponse optimization of an extraction procedure of carnosol and rosmarinic and carnosic acids from rosemary. Food Chem. 2016, 211, 465–473.
- Max, B.; Salgado, J.M.; Cortés, S.; Domínguez, J.M. Extraction of phenolic acids by alkaline hydrolysis from the solid residue obtained after prehydrolysis of trimming vine shoots. J. Agric. Food Chem. 2009, 58, 1909–1917.
- Gonzales, G.B.; Raes, K.; Vanhoutte, H.; Coelus, S.; Smagghe, G.; Van Camp, J. Liquid chromatography–mass spectrometry coupled with multivariate analysis for the characterization and discrimination of extractable and nonextractable polyphenols and glucosinolates from red cabbage and Brussels sprout waste streams. J. Chrom. A 2015, 1402, 60–70.
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370.
- Kehan, P.; Ou, J.; Huanga, J.; Oua, S. Coumaric acid and itsconjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962.
- Proestos, C.; Komaitis, M. Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT-Food Sci. Technol. 2008, 41, 652–659.
- Bernatoniene, J.; Cizauskaite, U.; Ivanauskas, L.; Jakstas, V.; Kalveniene, Z.; Kopustinskiene, D.M. Novel approaches to optimize extraction processes of ursolic, oleanolic and rosmarinic acids from Rosmarinus officinalis leaves. Ind. Crops Prod. 2016, 84, 72–79.
- Ciulu, M.; de la Luz Cádiz-Gurrea, M.; Segura-Carretero, A. Extraction and Analysis of Phenolic Compounds in Rice: A Review. Molecules 2018, 23, 2890.
- Pyrzynska, K.; Sentkowska, A. Chromatographic Analysis of Polyphenols. In Polyphenols in Plants Isolation Purification and Extract Preparation, 2nd ed.; Watson, R.R., Ed.; Elsevier Inc.: London, UK, 2019; pp. 353–364.
- Vinas, P.; Campillo, N. Gas Chromatography: Mass Spectrometry Analysis of Polyphenols in Foods. In Polyphenols in Plants Isolation Purification and Extract Preparation, 2nd ed.; Watson, R.R., Ed.; Elsevier Inc.: London, UK, 2019; pp. 285–316.
This entry is offline, you can click here to edit this entry!
