Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
|
Cell & Tissue Engineering
Four sensory systems (vestibular, lateral line, electroreception, auditory) are unique and project exclusively to the brainstem of vertebrates. All sensory neurons depend on a common set of genes (Eya1, Sox2, Neurog1, Neurod1) that project to a dorsal nucleus and an intermediate nucleus, which differentiate into the vestibular ear, lateral line and electroreception in vertebrates.
- neurons
- brainstem nuclei
- hair cells
- bHLH genes
- Sox2
- Eya1
- Lmx1a/b
1. Introduction
Sensory maps depend on the specific sensory modality and the relevant information to be extracted by them. Beyond primary sensory maps, central map formation underlies the integration of various sensory modalities, namely the ear, lateral line and electroreception. The four primary sensory maps of vertebrates have unique features and seemingly use distinct molecular cues, cell cycle exit and activity combinations during development, regeneration and plasticity. The evolution of chordates is comparable with the organization of the dorsal spinal cord and brainstem, which is associated with neurons and hair cells in 71,000 vertebrates. On the other hand, we have limited support for the two chordates associated with the neural crest and placodes, hair cells and central brainstem in 31 species of lancelets and 3100 species of ascidians. Fossils appeared approximately 540 million years ago (Mya), and all major bilaterian phyla presented by 500 Mya [1].
The brainstem of vertebrates is organized into rhombomeres (r0-11) that superficially resemble other chordates, lancelet and ascidians [2][3][4]. A dorsal part of the brainstem expresses a continuation to the spinal cord in vertebrates [5] which is absent in a true brainstem in other chordates. Partial similarity is found in ‘dorsal root ganglia’ in ascidians that resembles the spinal cord in vertebrates, which is absent in lancelets [2][6][7]. Adding these differences in chordates, gene duplication [8], followed by diversification [9][10], is the basis for the unique brainstem, neurons and hair cells that developed in vertebrates [11]. The unique formation of mechano- and electroreception evolved in four distinct sensory inputs that are partially similar with the lateral line of ascidians [6][12][13][14]. The progression must start with the sensory neurons that connect all neurons with the brainstem and reach out the peripheral sensory hair cells.
Neurons depend upon Eya1 [15], Sox2 [16], Neurog1 [17] and Neurod1 [18]. In contrast to Neurog1 null mice, which showed a complete loss of neurons [19], Neurod1 null mice showed residual neurons extending centrally to smaller vestibular and cochlear nuclei [20][21] that reached the ear [22][23]. It is worth noting that the lateral line and electroreception are separate for the vertebrate ear that is lost in most tetrapods to generate novel cochlear neurons, the spiral ganglion neurons (Figure 1).
The brainstem is a continuation of the spinal cord (SC; [11][24][25]) that develops into rhombomeres and differentiates into nuclei, namely the vestibular, lateral line and electroreception nuclei in basal vertebrates (Figure 1). Loss of the lateral line and electroreception leads to the development of cochlear nuclei in tetrapods [26][27]. All dorsal expression of the brainstem depends on Lmx1a/b [28] and Gdf7 [29], which drive the choroid plexus (Figure 1). Combined, Lmx1a/b and Gdf7 regulate the formation of Wnt1/3a, BMP4/7 and Atoh1. This formation is likely reduced or absent in Neurog1/2, Ascl1, Ptf1a and Olig3, among others (Figure 1).
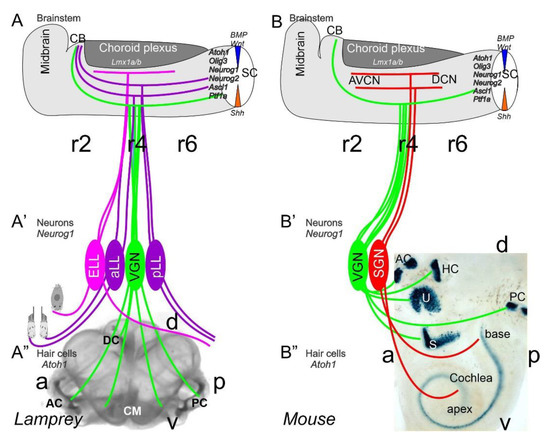

Figure 1. Inner ear, lateral line and electroreception revealed. Neurons (Neurog1; A’) form vestibular ganglia (VGN) to reach out 4 hair cell organs in lampreys (A”). A separate lateral line (LL) and electroreceptor neurons (ELL) that innervate hair cells project more dorsal in lampreys. Central projection depends on Atoh1 to receive LL and ELL fibers, whereas several bHLH genes (Neurog1/2, Olig3, Ascla1, Ptf1a) receive all VGN (A). In the absence of ELL and LL development in amniotes, mammals develop separate spiral ganglion neurons (SGN; B’) that extend from the cochlea (B”) and end in a topological central projection that depends on Atoh1 (B). The formation of VGNs (Neurog1; B’) reach the 5 hair cells (B”) to extend the distribution of bHLH genes. Note that certain areas are lost or gained which enter central projections near r4. Images are shown by miR-183 ISH (A”) and Atoh1-LacZ (B”). AC, anterior crista; AVCN, anteroventral cochlear neurons; CB, cerebellum; aLL, pLL, anterior/posterior lateral line neurons; CM, common macula; DC, dorsal crista; DCN, dorsal cochlear neurons; HC, horizontal crista; PC, posterior crista; r2/4/6, rhombomeres; S, saccule; SC, spinal cord; U, utricle. Modified after [11][30][31].
Mechanosensory and electrosensory hair cells (Figure 1) depend on Eya1, Sox2 and Atoh1 to initiate the cell cycle and to differentiate into vestibular, cochlear, lateral line and electrosensory hair cells [22][32][33]. Planar cell polarity (PCP) depends on the formation of shifting the central projection of the kinocilium into a lateral position. PCP extends the length of the stereocilia to develop the staircase of tip links of the vestibular, cochlear and lateral line hair cells [34][35][36]. The next step involves the development of the tip links to allow the connections between CDH23 and PCDH15 to open up the channel to form a mechanosensory hair cell [37][38], with opposing polarity in most of the ear and lateral line [34][39][40][41]. TMC1/2 provides a major function that seems to interact with additional channel proteins (TMHS, TMIE), forming a complex interaction [37][42][43][44]. In contrast, while the electroreception forms next to lateral line hair cells [22][23][45], these hair cells lack any polarity organization, and certain ampullary hair cells are dependent on Cav1.3 [46].
2. Neurons Depend upon Eya1, Sox2, Neurog1 and Neurod1
The ear, lateral line and electroreception neurons depend on genes that, collectively, define their development. Upstream of bHLH genes, which initiate the proliferation of neurons, is the expression of Eya1, which interacts with Brg1 to initiate pro-neurosensory development [15][47][48]. In the absence of Eya1, there is no neuronal development that allows ear formation, and neither neurons nor hair cells differentiate [15]. Evolving neurons start in the lancelet, which lack dorsal root ganglia. The dorsal root ganglia show partial expression of Neurog inside the spinal cord (Figure 2), which lacks an Atoh gene [49][50]. In contrast, at least a smaller set of bHLH genes are partially characterized in the developing ascidian, Ciona [51], which have at least six bHLH genes driving neuron development: Ptf1a, Tcf3, Atoh, Ascl and Neurog [7][12]. A detailed serial section analysis shows the innervation of sensory cells (Atoh) from fibers of the neurons (bipolar tail neurons; Figure 2) that can trace to reach the anterior motor ganglion [13]. Neither the full expression of Eya nor Sox2 outside the neural plate are unclear in the lancelet and tunicates [2][51].
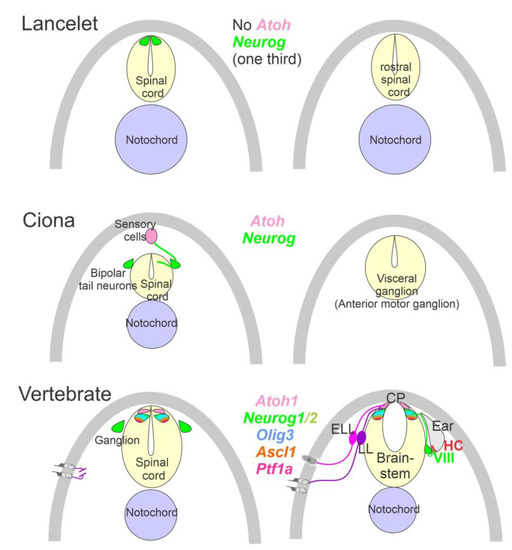
Figure 2. Neurons require Neurog expression. Lancelets have a limited description of bHLH genes that are characterized in the more caudal spinal cord, which is positive for Neurog. Note that the lancelet has no Atoh bHLH gene. Ciona has at least 6 bHLH genes expressed in sensory cells that are innervated by bipolar tail neurons which extend to reach the visceral ganglion for interactions. Atoh and Neurog genes are described in Ciona associated with the spinal cord. Vertebrates have dorsal root ganglia that depend on Neurog1/2, which is also expressed in Atoh1 and Neurog1 of the spinal cord. The brainstem is innervated by electroreceptor (ELL) and lateral line fibers (LL) that extend to innervate migration populations of LL and some ELL). The ear is unique in vertebrates, which give rise to the VIII ganglia that innervate more ventral nuclei compared to LL and ELL projections to reach Atoh1. CP, choroid plexus. Modified after [2][7][12][23][24].
A crucial next step is the initiation of Sox2, which is needed to upregulate Neurog1 [52][53][54]. In fact, Sox2 delays certain neuron development in bony fish [55], and in the presence of Sox2 is unclear the sequence of gene regulation in the lamprey and hagfish [56]. There is a distinct effect of the loss of early genes in the vestibular ganglion, which initially differentiates in the absence of Sox2 and Neurog1 (Figure 1 and Figure 2) and does not develop in the auditory neurons [16]. A loss of all auditory neurons, and partial loss of vestibular neurons, are known for Pax2 [57], Gata3 [58], Lmx1a/b [28], Fgfr2 [59], Shh [60] and Dicer [61]. Partial loss of some vestibular neurons are known for Fgf10 [62] and Foxg1 [63][64], indicating a limited loss of sensory hair cells and/or neurons. Unfortunately, the details of the lateral line and electroreception (Figure 1, Figure 2 and Figure 3) are not as fully genetically characterized [22][23][27][33]. The lateral line and electroreception likely depend on neuronal development (Figure 1 and Figure 2), including the development of spinal ganglia neurons [65] and trigeminal neurons [66][67][68]. A separate placode is derived from neurons that develop from Neurog2 in mammals [67][69]. In birds, this placode is driven by Neurog1 [70][71]. Furthermore, separate amniotic paratympanic placodal neurons innervate separate hair cells that partially integrate into the central vestibular projection [71].
In addition to directly initiating the formation of neurons by Eya1, Sox2, Pax2 and Neurog1/2, another set of genes are regulated to differentiate into Neurod1 [18][20][21][70][72], followed by Isl1, Foxg1, Pou4f1 and Phox2b [70][73][74][75], which interact with Shh, BMPs and Wnts to define neurons [76][77]. Regional regulation of the distinct vestibular, lateral line, electroreception and auditory neurons are sorted out by downstream genes regulating the distinct innervation. For example, the expression of Calbindin, Calretinin, Pou4f1 and Peripherin is required to sort out the innervation from the inner and outer hair cells [78][79][80][81]. In Sox10 null mice, an interaction showed disorganized cochlear neurons, whereas the development of vestibular neurons was near normal [82]. This interaction is consistent with the loss of Erb2 of nearly all cochlear neurons, as well as reduced vestibular neurons [83]. The concept of having multiple sources of neurons from the placode and neural crest is likely due to a misinterpretation [3][82][84][85][86].
Downstream of gene development, the expression of TrkB (Ntrk2) and TrkC (Ntrk3) has a reduction and loss in vestibular and cochlear neurons. Vestibular neurons are mostly dependent on TrkB [87][88] whereas the cochlear neurons are mostly dependent on TrkC [89][90]. Loss of both neurotrophin receptors causes the early loss of all neurons [91][92][93]. Limited expression is characterized in some ascidians which are unknown in the lancelet [1]. The comparable expression of the lateral line and electroreception are unclear due to the multiplication of neurotrophins in bony fish [94][95].
The proliferation of neurons and hair cells depend on MycN [96][97], which drives the division of the G1, S and G2 phases with a set of genes that interactions with cell cycle regulation [52][98][99][100]. Detailed characterization and proliferation have been described in the ear and brainstem, clarifying cell cycle progression in mice and rats [101][102][103]. Sox2 and Neurog1 are in negative feedback, which allows proliferation and initiates differentiation. This differentiation interacts with retinoblastoma (Rb), Hes/Hey and IDs to regulate the cyclin-dependent kinases (CDKs), cross-react with e-proteins and define whether a cell cycle is progressing [97][99][104][105]. In the end, continuation depends on either knocking out Rb to continue proliferation or upregulating of Sox2 to jumpstart proliferation [106][107].
In various vertebra, the central projection has been described to show the projection of the vestibular, lateral line, electroreception, and cochlea [3][66][86][108][109][110]. Three sets of central projections are known in vertebrates that develop a loss of the lateral line, electroreception and added cochlear nuclei [23][26][111]. For electroreception, these central projections always have a single set of an anterior ganglia (Figure 1 and Figure 3) that adds variably the electroreception in bony fish [27][112]. Lateral line neurons (Figure 1, Figure 2 and Figure 3) can be split into an anterior and posterior branch that diversify the neuromasts to innervate all lateral line hair cells (Figure 3; [113][114][115]). Vestibular neurons have two neuron populations in hagfish [56], while lampreys and jawed vertebrates have a single vestibular ganglion [110][116][117]. At least 4-5 distinct innervations are described in lampreys [118][119], whereas most gnathostomes have at least five and up to nine branches of vestibular and auditory connections (Figure 1 and Figure 3): three canal cristae, utricle, saccule, lagena, basilar papilla, amphibian papilla and neglecta [120][121]. Branches of discrete neurons are known for an anterior and a posterior (superior) nucleus that innervates two canal cristae (anterior and horizontal cristae), the utricle and part of the saccule (Figure 3). The remaining part of the utricle provides a posterior canal and the branch of the saccule (Figure 1 and Figure 3) in mammals [122]. The development of central projections follows a simple layout. First, the trigeminal and epibranchial neurons develop. Then, central projection follows. Subsequently, vestibular, lateral line and electroception develop, if present (Figure 3; [3][123]). Different developmental patterns exist in neuronal proliferation: nearly all neurons continue proliferation for a long time or lifetime, whereas mammals have an early production of neurons that ends proliferation very early [66][124][125]. The topology of peripheral neurons of the vestibular, lateral line and electroreceptors is unclear, suggesting an overlap with an incomplete segregation of neurons that is well known for the vestibular neurons (Figure 3 [122]).
A long-term proliferation of the vestibular, lateral line and electroreception is followed by a delayed formation of cochlear neurons, the spiral ganglia neurons (SGN), which follow vestibular neurons in mammals (vestibular neurons: E9-11; SGN: E10-12 [124][126]). A unique topological development is known among mammals [127], first showing the basal turn neurons (Figure 3), which reach the anteroventral, posteroventral, and dorsal cochlear nuclei (AVCN, PVCN, DCN). The development of these neurons is followed, with delay, by the apical neurons [66][86][109][128]. Interestingly, there are central projections that can form independently to reach the formation of cochlear nuclei [129]. In the absence of target hair cell development [91][130], cochlear neurons develop and largely proliferate prior to cochlear nuclei and cochlear hair cells (Figure 3). Central cochlea require the expression of Neurod1, Wnts, Fzd, Npr2 and Ephrins for targeted central projections [21][128][131][132].
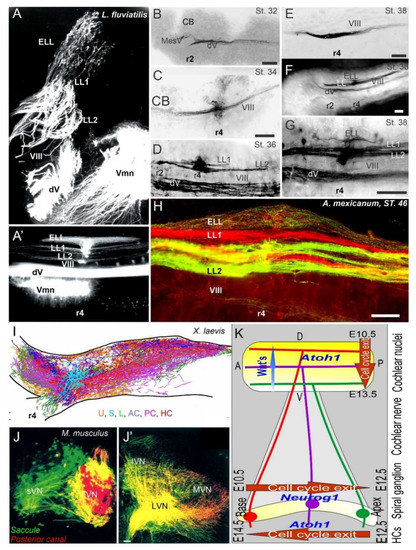

Figure 3. Central projections form afferents to distinct innervation. The lateral line of 2 or more branches form, whereas electroreception receives the short dorsal projection in lampreys (A,A’) and salamanders (B–H). Vestibular projection forms after the trigeminal central projection, followed by the lateral line and electroreception (B–H). Central projection in a frog (I) and mammal (J,J’) show the incomplete distribution of distinct neurons (J) that overlap and incompletely segregate the vestibular projection (I,J’). Spiral ganglia (K) proliferate neurons in a base to apex progression (E10.5-12.5) that reach the central projection to form a topology from dorsal to ventral cochlear nuclei (E10.5-13.5), depending on Wnt expression. Later, hair cells proliferate from apex to base (E12.5-14.5) that reach the afferents. AC, anterior crista; dV, trigeminal afferents; ELL, electroreception; HC, horizontal crista; LL1/2; lateral line; L, lagena; LVN, lateral vestibular nuclei; IVN, inferior vestibular nuclei; iVN, inferior vestibular neurons; MVN, medial vestibular nuclei; PC, posterior crista; S, saccule; sVN, superior vestibular neurons; U, utricle; Vmn, trigeminal motoneurons; VIII, vestibular projections. Modified after [3][23][66][122].
In contrast to the topology of the cochlear nuclei [11][127], the central vestibular neurons have an incomplete central segregation (Figure 3) that shows both segregation and overlap from different vestibular neurons [3][122][133]. Lateral line central projections can be segregated in certain vertebrates but show an overlap in other vertebrates [3][23]. For electroreception, multiple central topological projections in certain bony fish [27][134] show an overlap in lampreys and salamanders (Figure 3 [23][108]). The vestibular, lateral line, electroreception and cochlea independently reach hair cells that form prior to neurons [23][135], consistent with the same pattern of neurons that develop first, followed by the central axon to the brainstem, and later followed by the hair cell innervation [3][108][133][136]. This is obvious in cases where hair cells are not formed, such as in Atoh1 null mice, which show a near-normal central projection [130][137]. A similar central projection forms after the loss of hair cells in Pou4f1 null mice [138]. Loss of formation of a specific set of hair cells is demonstrated in the posterior canal that projects normally, despite the absence of Fgf10 [62], which degenerates later.
In summary, the neurons of the ear, lateral line and electroreception are generated by a set of genes that act downstream of Neurog1 to initiate the cell cycle. Neurons develop independently of central axons and reach innervate the hair cells shortly after proliferation. Segregation of central projections can be topologically organized in the auditory central projection of most tetrapods, and present two lateral line neurons that segregated in many vertebrates. Some central topology found in some, but not all, lateral line and electroreceptors, show an incomplete segregation for the vestibular neurons.
This entry is adapted from the peer-reviewed paper 10.3390/d13080364
References
- Heger, P.; Zheng, W.; Rottmann, A.; Panfilio, K.A.; Wiehe, T. The genetic factors of bilaterian evolution. eLife 2020, 9, e45530.
- Holland, L. Invertebrate origins of vertebrate nervous systems. In Evolutionary Neuroscience; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–73.
- Chagnaud, B.P.; Engelmann, J.; Fritzsch, B.; Glover, J.C.; Straka, H. Sensing external and self-motion with hair cells: A comparison of the lateral line and vestibular systems from a developmental and evolutionary perspective. Brain Behav. Evol. 2017, 90, 98–116.
- Fritzsch, B.; Elliott, K.L.; Glover, J.C. Gaskell revisited: New insights into spinal autonomics necessitate a revised motor neuron nomenclature. Cell Tissue Res. 2017, 370, 195–209.
- Bermingham, N.A.; Hassan, B.A.; Wang, V.Y.; Fernandez, M.; Banfi, S.; Bellen, H.J.; Fritzsch, B.; Zoghbi, H.Y. Proprioceptor pathway development is dependent on Math1. Neuron 2001, 30, 411–422.
- Kim, K.; Gibboney, S.; Razy-Krajka, F.; Lowe, E.K.; Wang, W.; Stolfi, A. Regulation of neurogenesis by FGF signaling and Neurogenin in the invertebrate chordate Ciona. Front. Cell Dev. Biol. 2020, 8, 477.
- Stolfi, A.; Ryan, K.; Meinertzhagen, I.A.; Christiaen, L. Migratory neuronal progenitors arise from the neural plate borders in tunicates. Nature 2015, 527, 371–374.
- Holland, L.Z.; Daza, D.O. A new look at an old question: When did the second whole genome duplication occur in vertebrate evolution? Genome Biol. 2018, 19, 1–4.
- Fritzsch, B.; Jahan, I.; Pan, N.; Elliott, K.L. Evolving gene regulatory networks into cellular networks guiding adaptive behavior: An outline how single cells could have evolved into a centralized neurosensory system. Cell Tissue Res. 2015, 359, 295–313.
- Fritzsch, B.; Elliott, K.L. Gene, cell, and organ multiplication drives inner ear evolution. Dev. Biol. 2017, 431, 3–15.
- Elliott, K.L.; Pavlínková, G.; Chizhikov, V.V.; Yamoah, E.N.; Fritzsch, B. Development in the Mammalian Auditory System Depends on Transcription Factors. Int. J. Mol. Sci. 2021, 22, 4189.
- Tang, W.J.; Chen, J.S.; Zeller, R.W. Transcriptional regulation of the peripheral nervous system in Ciona intestinalis. Dev. Biol. 2013, 378, 183–193.
- Ryan, K.; Lu, Z.; Meinertzhagen, I.A. The peripheral nervous system of the ascidian tadpole larva: Types of neurons and their synaptic networks. J. Comp. Neurol. 2018, 526, 583–608.
- Manni, L.; Anselmi, C.; Burighel, P.; Martini, M.; Gasparini, F. Differentiation and induced sensorial alteration of the coronal organ in the asexual life of a tunicate. Integr. Comp. Biol. 2018, 58, 317–328.
- Xu, J.; Li, J.; Zhang, T.; Jiang, H.; Ramakrishnan, A.; Fritzsch, B.; Shen, L.; Xu, P.X. Chromatin remodelers and lineage-specific factors interact to target enhancers to establish proneurosensory fate within otic ectoderm. Proc. Natl. Acad. Sci. USA 2021, 118, 1–12.
- Dvorakova, M.; Macova, I.; Bohuslavova, R.; Anderova, M.; Fritzsch, B.; Pavlinkova, G. Early ear neuronal development, but not olfactory or lens development, can proceed without SOX2. Dev. Biol. 2020, 457, 43–56.
- Ma, Q.; Chen, Z.; del Barco Barrantes, I.; de la Pompa, J.L.; Anderson, D.J. Neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron 1998, 20, 469–482.
- Kim, W.-Y.; Fritzsch, B.; Serls, A.; Bakel, L.A.; Huang, E.J.; Reichardt, L.F.; Barth, D.S.; Lee, J.E. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development 2001, 128, 417–426.
- Ma, Q.; Anderson, D.J.; Fritzsch, B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J. Assoc. Res. Otolaryngol. 2000, 1, 129–143.
- Jahan, I.; Kersigo, J.; Pan, N.; Fritzsch, B. Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell Tissue Res. 2010, 341, 95–110.
- Macova, I.; Pysanenko, K.; Chumak, T.; Dvorakova, M.; Bohuslavova, R.; Syka, J.; Fritzsch, B.; Pavlinkova, G. Neurod1 is essential for the primary tonotopic organization and related auditory information processing in the midbrain. J. Neurosci. 2019, 39, 984–1004.
- Baker, C.V. The Development and Evolution of Lateral Line Electroreceptors: Insights from Comparative Molecular Approaches. In Electroreception: Fundamental Insights from Comparative Approaches; Springer: Berlin/Heidelberg, Germany, 2019; pp. 25–62.
- Elliott, K.L.; Fritzsch, B. Evolution and development of lateral line and electroreception: An integrated perception ofneurons, hair cells and brainstem nuclei. In The Senses; Fritzsch, B., Bleckmann, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 7, pp. 95–115.
- Lai, H.C.; Seal, R.P.; Johnson, J.E. Making sense out of spinal cord somatosensory development. Development 2016, 143, 3434–3448.
- Hernandez-Miranda, L.R.; Müller, T.; Birchmeier, C. The dorsal spinal cord and hindbrain: From developmental mechanisms to functional circuits. Dev. Biol. 2017, 432, 34–42.
- Grothe, B.; Carr, C.E.; Casseday, J.H.; Fritzsch, B.; Köppl, C. The evolution of central pathways and their neural processing patterns. In Evolution of the Vertebrate Auditory System; Springer: Berlin/Heidelberg, Germany, 2004; pp. 289–359.
- Wullimann, M.F.; Grothe, B. The central nervous organization of the lateral line system. In The Lateral Line System; Springer: Berlin/Heidelberg, Germany, 2013; pp. 195–251.
- Chizhikov, V.V.; Iskusnykh, I.Y.; Fattakhov, N.; Fritzsch, B. Lmx1a and Lmx1b are Redundantly Required for the Development of Multiple Components of the Mammalian Auditory System. Neuroscience 2021, 452, 247–264.
- Lee, K.J.; Dietrich, P.; Jessell, T.M. Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature 2000, 403, 734–740.
- Pierce, M.L.; Weston, M.D.; Fritzsch, B.; Gabel, H.W.; Ruvkun, G.; Soukup, G.A. MicroRNA—183 family conservation and ciliated neurosensory organ expression. Evol. Dev. 2008, 10, 106–113.
- Nichols, D.H.; Pauley, S.; Jahan, I.; Beisel, K.W.; Millen, K.J.; Fritzsch, B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008, 334, 339–358.
- Arendt, D.; Musser, J.M.; Baker, C.V.; Bergman, A.; Cepko, C.; Erwin, D.H.; Pavlicev, M.; Schlosser, G.; Widder, S.; Laubichler, M.D. The origin and evolution of cell types. Nat. Rev. Genet. 2016, 17, 744–757.
- Schlosser, G. A short history of nearly every sense—The evolutionary history of vertebrate sensory cell types. Integr. Comp. Biol. 2018, 58, 301–316.
- Tarchini, B.; Lu, X. New insights into regulation and function of planar polarity in the inner ear. Neurosci. Lett. 2019, 709, 134373.
- Montcouquiol, M.; Rachel, R.A.; Lanford, P.J.; Copeland, N.G.; Jenkins, N.A.; Kelley, M.W. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 2003, 423, 173–177.
- Ghimire, S.R.; Deans, M.R. Frizzled3 and Frizzled6 Cooperate with Vangl2 to Direct Cochlear Innervation by type II Spiral Ganglion Neurons. J. Neurosci. 2019, 39, 8013–8023.
- Qiu, X.; Müller, U. Mechanically gated ion channels in mammalian hair cells. Front. Cell. Neurosci. 2018, 12, 100.
- Shibata, S.B.; Ranum, P.T.; Moteki, H.; Pan, B.; Goodwin, A.T.; Goodman, S.S.; Abbas, P.J.; Holt, J.R.; Smith, R.J. RNA interference prevents autosomal-dominant hearing loss. Am. J. Hum. Genet. 2016, 98, 1101–1113.
- Fritzsch, B.; López-Schier, H. Evolution of polarized hair cells in aquatic vertebrates and their connection to directionally sensitive neurons. In Flow Sensing in Air and Water; Springer: Berlin/Heidelberg, Germany, 2014; pp. 271–294.
- Kozak, E.L.; Palit, S.; Miranda-Rodriguez, J.R.; Janjic, A.; Bottcher, A.; Lickert, H.; Enard, W.; Theis, F.J.; Lopez-Schier, H. Epithelial Planar Bipolarity Emerges from Notch-Mediated Asymmetric Inhibition of Emx2. Curr. Biol. 2020, 30, 1142–1151.e6.
- Jiang, T.; Kindt, K.; Wu, D.K. Transcription factor Emx2 controls stereociliary bundle orientation of sensory hair cells. eLife 2017, 6.
- Pan, B.; Akyuz, N.; Liu, X.P.; Asai, Y.; Nist-Lund, C.; Kurima, K.; Derfler, B.H.; Gyorgy, B.; Limapichat, W.; Walujkar, S.; et al. TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells. Neuron 2018, 99, 736–753.e6.
- Erives, A.; Fritzsch, B. A Screen for Gene Paralogies Delineating Evolutionary Branching Order of Early Metazoa. G3 Genes Genom. Genet. 2020, 10, 811–826.
- Schwander, M.; Kachar, B.; Müller, U. The cell biology of hearing. J. Cell Biol. 2010, 190, 9–20.
- Northcutt, R.G.; Brändle, K.; Fritzsch, B. Electroreceptors and mechanosensory lateral line organs arise from single placodes in axolotls. Dev. Biol. 1995, 168, 358–373.
- Leitch, D.B.; Julius, D. Electrosensory Transduction: Comparisons Across Structure, Afferent Response Properties, and Cellular Physiology. In Electroreception: Fundamental Insights from Comparative Approaches; Springer: Berlin/Heidelberg, Germany, 2019; pp. 63–90.
- Xu, P.X.; Adams, J.; Peters, H.; Brown, M.C.; Heaney, S.; Maas, R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat. Genet. 1999, 23, 113–117.
- Zou, D.; Silvius, D.; Fritzsch, B.; Xu, P.-X. Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development 2004, 131, 5561–5572.
- Holland, N.D.; Somorjai, I.M. The sensory peripheral nervous system in the tail of a cephalochordate studied by serial blockface scanning electron microscopy. J. Comp. Neurol. 2020, 528, 2569–2582.
- Fritzsch, B.; Northcutt, G. Cranial and spinal nerve organization in amphioxus and lampreys: Evidence for an ancestral craniate pattern. Cells Tissues Organs 1993, 148, 96–109.
- Negrón-Piñeiro, L.J.; Wu, Y.; Di Gregorio, A. Transcription Factors of the bHLH Family Delineate Vertebrate Landmarks in the Nervous System of a Simple Chordate. Genes 2020, 11, 1262.
- Kageyama, R.; Shimojo, H.; Ohtsuka, T. Dynamic control of neural stem cells by bHLH factors. Neurosci. Res. 2019, 138, 12–18.
- Reiprich, S.; Wegner, M. From CNS stem cells to neurons and glia: Sox for everyone. Cell Tissue Res. 2015, 359, 111–124.
- Riddiford, N.; Schlosser, G. Dissecting the pre-placodal transcriptome to reveal presumptive direct targets of Six1 and Eya1 in cranial placodes. eLife 2016, 5, e17666.
- Millimaki, B.B.; Sweet, E.M.; Riley, B.B. Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev. Biol. 2010, 338, 262–269.
- Higuchi, S.; Sugahara, F.; Pascual-Anaya, J.; Takagi, W.; Oisi, Y.; Kuratani, S. Inner ear development in cyclostomes and evolution of the vertebrate semicircular canals. Nature 2019, 565, 347–350.
- Bouchard, M.; de Caprona, D.; Busslinger, M.; Xu, P.; Fritzsch, B. Pax2 and Pax8 cooperate in mouse inner ear morphogenesis and innervation. BMC Dev. Biol. 2010, 10, 89.
- Duncan, J.S.; Fritzsch, B. Continued expression of GATA3 is necessary for cochlear neurosensory development. PLoS ONE 2013, 8, e62046.
- Pirvola, U.; Spencer-Dene, B.; Xing-Qun, L.; Kettunen, P.; Thesleff, I.; Fritzsch, B.; Dickson, C.; Ylikoski, J. FGF/FGFR-2 (IIIb) signaling is essential for inner ear morphogenesis. J. Neurosci. 2000, 20, 6125–6134.
- Riccomagno, M.M.; Martinu, L.; Mulheisen, M.; Wu, D.K.; Epstein, D.J. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002, 16, 2365–2378.
- Kersigo, J.; D’Angelo, A.; Gray, B.D.; Soukup, G.A.; Fritzsch, B. The role of sensory organs and the forebrain for the development of the craniofacial shape as revealed by Foxg1-cre-mediated microRNA loss. Genesis 2011, 49, 326–341.
- Pauley, S.; Wright, T.J.; Pirvola, U.; Ornitz, D.; Beisel, K.; Fritzsch, B. Expression and function of FGF10 in mammalian inner ear development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2003, 227, 203–215.
- Pauley, S.; Lai, E.; Fritzsch, B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2006, 235, 2470–2482.
- Hwang, C.H.; Simeone, A.; Lai, E.; Wu, D.K. Foxg1 is required for proper separation and formation of sensory cristae during inner ear development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2009, 238, 2725–2734.
- Ma, Q.; Fode, C.; Guillemot, F.; Anderson, D.J. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999, 13, 1717–1728.
- Fritzsch, B.; Elliott, K.L.; Pavlinkova, G. Primary sensory map formations reflect unique needs and molecular cues specific to each sensory system. F1000Research 2019, 8, 345.
- Dennis, D.J.; Han, S.; Schuurmans, C. bHLH transcription factors in neural development, disease, and reprogramming. Brain Res. 2019, 1705, 48–65.
- Erzurumlu, R.S.; Murakami, Y.; Rijli, F.M. Mapping the face in the somatosensory brainstem. Nat. Rev. Neurosci. 2010, 11, 252–263.
- Fode, C.; Gradwohl, G.; Morin, X.; Dierich, A.; Le Meur, M.; Goridis, C.; Guillemot, F. The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode–derived sensory neurons. Neuron 1998, 20, 483–494.
- Alsina, B. Mechanisms of cell specification and differentiation in vertebrate cranial sensory systems. Curr. Opin. Cell Biol. 2020, 67, 79–85.
- O'Neill, P.; Mak, S.-S.; Fritzsch, B.; Ladher, R.K.; Baker, C.V. The amniote paratympanic organ develops from a previously undiscovered sensory placode. Nat. Commun. 2012, 3, 1041.
- Sapede, D.; Dyballa, S.; Pujades, C. Cell lineage analysis reveals three different progenitor pools for neurosensory elements in the otic vesicle. J. Neurosci. 2012, 32, 16424–16434.
- Moody, S.A.; La Mantia, A.-S. Transcriptional regulation of cranial sensory placode development. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 111, pp. 301–350.
- Dykes, I.M.; Tempest, L.; Lee, S.-I.; Turner, E.E. Brn3a and Islet1 act epistatically to regulate the gene expression program of sensory differentiation. J. Neurosci. 2011, 31, 9789–9799.
- Huang, E.J.; Liu, W.; Fritzsch, B.; Bianchi, L.M.; Reichardt, L.F.; Xiang, M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development 2001, 128, 2421–2432.
- Mackowetzky, K.; Yoon, K.H.; Mackowetzky, E.J.; Waskiewicz, A.J. Development and evolution of the vestibular apparatuses of the inner ear. J. Anat. 2021.
- Muthu, V.; Rohacek, A.M.; Yao, Y.; Rakowiecki, S.M.; Brown, A.S.; Zhao, Y.-T.; Meyers, J.; Won, K.-J.; Ramdas, S.; Brown, C.D.; et al. Genomic architecture of Shh-dependent cochlear morphogenesis. Development 2019, 146, dev181339.
- Petitpré, C.; Wu, H.; Sharma, A.; Tokarska, A.; Fontanet, P.; Wang, Y.; Helmbacher, F.; Yackle, K.; Silberberg, G.; Hadjab, S. Neuronal heterogeneity and stereotyped connectivity in the auditory afferent system. Nat. Commun. 2018, 9, 3691.
- Shrestha, B.R.; Chia, C.; Wu, L.; Kujawa, S.G.; Liberman, M.C.; Goodrich, L.V. Sensory neuron diversity in the inner ear is shaped by activity. Cell 2018, 174, 1229–1246.e17.
- Sun, S.; Babola, T.; Pregernig, G.; So, K.S.; Nguyen, M.; Su, S.-S.M.; Palermo, A.T.; Bergles, D.E.; Burns, J.C.; Müller, U. Hair cell mechanotransduction regulates spontaneous activity and spiral ganglion subtype specification in the auditory system. Cell 2018, 174, 1247–1263.e15.
- Elliott, K.L.; Kersigo, J.M.; Lee, J.H.; Jahan, I.; Pavlinkova, G.; Fritzsch, B.; Yamoah, E.N. Changes in Peripherin-eGFP expression in spiral ganglion neurons; from development to maturity. Front. Cell. Neurosci. 2021, 15, 181.
- Mao, Y.; Reiprich, S.; Wegner, M.; Fritzsch, B. Targeted deletion of Sox10 by Wnt1-cre defects neuronal migration and projection in the mouse inner ear. PLoS ONE 2014, 9, e94580.
- Morris, J.K.; Maklad, A.; Hansen, L.A.; Feng, F.; Sorensen, C.; Lee, K.-F.; Macklin, W.B.; Fritzsch, B. A disorganized innervation of the inner ear persists in the absence of ErbB2. Brain Res. 2006, 1091, 186–199.
- Freyer, L.; Aggarwal, V.; Morrow, B.E. Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development 2011, 138, 5403–5414.
- Billings, S.E.; Myers, N.M.; Quiruz, L.; Cheng, A.G. Opposing effects of Wnt/β-catenin signaling on epithelial and mesenchymal cell fate in the developing cochlea. Development 2021, 148, dev199091.
- Goodrich, L.V. Early development of the spiral ganglion. In The Primary Auditory Neurons of the Mammalian Cochlea; Springer: Berlin/Heidelberg, Germany, 2016; pp. 11–48.
- Fritzsch, B.; Tessarollo, L.; Coppola, E.; Reichardt, L.F. Neurotrophins in the ear: Their roles in sensory neuron survival and fiber guidance. Prog. Brain Res. 2004, 146, 265–278.
- Bianchi, L.M.; Conover, J.C.; Fritzsch, B.; De Chiara, T.; Lindsay, R.M.; Yancopoulos, G.D. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development 1996, 122, 1965–1973.
- Fritzsch, B.; Kersigo, J.; Yang, T.; Jahan, I.; Pan, N. Neurotrophic factor function during ear development: Expression changes define critical phases for neuronal viability. In The Primary Auditoryneurons of the Mammaliancochlea; Dabdoub, A., Fritzsch, B., Popper, A., Fay, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 52, pp. 49–84.
- Fariñas, I.; Jones, K.R.; Tessarollo, L.; Vigers, A.J.; Huang, E.; Kirstein, M.; De Caprona, D.C.; Coppola, V.; Backus, C.; Reichardt, L.F. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J. Neurosci. 2001, 21, 6170–6180.
- Kersigo, J.; Fritzsch, B. Inner ear hair cells deteriorate in mice engineered to have no or diminished innervation. Front. Aging Neurosci. 2015, 7, 33.
- Fritzsch, B.; Sarai, P.; Barbacid, M.; Silos-Santiago, I. Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. Int. J. Dev. Neurosci. 1997, 15, 563–576.
- Silos-Santiago, I.; Fagan, A.M.; Garber, M.; Fritzsch, B.; Barbacid, M. Severe sensory deficits but normal CNS development in newborn mice lacking TrkB and TrkC tyrosine protein kinase receptors. Eur. J. Neurosci. 1997, 9, 2045–2056.
- Hallböök, F.; Wilson, K.; Thorndyke, M.; Olinski, R.P. Formation and evolution of the chordate neurotrophin and Trk receptor genes. Brain Behav. Evol. 2006, 68, 133–144.
- Bothwell, M. Ngf, bdnf, nt3, and nt4. In Neurotrophic Factors; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–15.
- Kopecky, B.; Santi, P.; Johnson, S.; Schmitz, H.; Fritzsch, B. Conditional deletion of N-Myc disrupts neurosensory and non-sensory development of the ear. Dev. Dyn. 2011, 240, 1373–1390.
- Schimmang, T.; Pirvola, U. Coupling the cell cycle to development and regeneration of the inner ear. Semin. Cell Dev. Biol. 2013, 24, 507–513.
- Sakamoto, S.; Tateya, T.; Omori, K.; Kageyama, R. Id genes are required for morphogenesis and cellular patterning in the developing mammalian cochlea. Dev. Biol. 2020, 460, 164–175.
- Chen, S.-D.; Yang, J.-L.; Lin, Y.-C.; Chao, A.; Yang, D.-I. Emerging Roles of Inhibitor of Differentiation-1 in Alzheimer’s Disease: Cell Cycle Reentry and Beyond. Cells 2020, 9, 1746.
- Baker, N.E.; Brown, N.L. All in the family: Proneural bHLH genes and neuronal diversity. Development 2018, 145, dev159426.
- Pierce, E.T. Histogenesis of the dorsal and ventral cochlear nuclei in the mouse. An autoradiographic study. J. Comp. Neurol. 1967, 131, 27–53.
- Chou, S.-W.; Chen, Z.; Zhu, S.; Davis, R.W.; Hu, J.; Liu, L.; Fernando, C.A.; Kindig, K.; Brown, W.C.; Stepanyan, R. A molecular basis for water motion detection by the mechanosensory lateral line of zebrafish. Nat. Commun. 2017, 8, 2234.
- Altman, J.; Bayer, S.A. Atlas Of Prenatal Rat Brain Development; CRC Press: Boca Raton, FL, USA, 1995.
- Kopecky, B.; Fritzsch, B. The Myc road to hearing restoration. Cells 2012, 1, 667–698.
- Alonso, M.B.D.; Hernandez, I.L.; de la Fuente, M.A.; Garcia-Sancho, J.; Giraldez, F.; Schimmang, T. Transcription factor induced conversion of human fibroblasts towards the hair cell lineage. PLoS ONE 2018, 13, e0200210.
- Mantela, J.; Jiang, Z.; Ylikoski, J.; Fritzsch, B.; Zacksenhaus, E.; Pirvola, U. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development 2005, 132, 2377–2388.
- Yamoah, E.N.; Li, M.; Shah, A.; Elliott, K.L.; Cheah, K.; Xu, P.X.; Phillips, S.; Young, S.M., Jr.; Eberl, D.F.; Fritzsch, B. Using Sox2 to alleviate the hallmarks of age-related hearing loss. Ageing Res. Rev. 2020, 59, 101042.
- Fritzsch, B.; Gregory, D.; Rosa-Molinar, E. The development of the hindbrain afferent projections in the axolotl: Evidence for timing as a specific mechanism of afferent fiber sorting. Zoology 2005, 108, 297–306.
- Yang, T.; Kersigo, J.; Jahan, I.; Pan, N.; Fritzsch, B. The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hear. Res. 2011, 278, 21–33.
- Pflieger, J.F.; Dubuc, R. Relationship between vestibular primary afferents and vestibulospinal neurons in lampreys. J. Comp. Neurol. 2000, 427, 255–273.
- Duncan, J.S.; Cox, B.C. Anatomy and Development of the Inner Ear. In The Senses; Fritzsch, B., Grothe, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 253–276.
- Bell, C.C.; Han, V.; Sawtell, N.B. Cerebellum-like structures and their implications for cerebellar function. Annu. Rev. Neurosci. 2008, 31, 1–24.
- Northcutt, R.G. The phylogenetic distribution and innervation of craniate mechanoreceptive lateral lines. In The Mechanosensory Lateral Line; Springer: Berlin/Heidelberg, Germany, 1989; pp. 17–78.
- Webb, J.F. Morphology of the mechanosensory lateral lien system of fishes. In The Senses; Fritzsch, B., Bleckmann, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 7, pp. 29–46.
- Chitnis, A.B. Development of the zebrafish posterior lateral line system. In The Senses; Fritzsch, B., Bleckmann, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 7, pp. 66–84.
- Fritzsch, B.; Beisel, K.; Jones, K.; Farinas, I.; Maklad, A.; Lee, J.; Reichardt, L. Development and evolution of inner ear sensory epithelia and their innervation. J. Neurobiol. 2002, 53, 143–156.
- Pombal, M.A.; Megías, M. Development and functional organization of the cranial nerves in lampreys. Anat. Rec. 2019, 302, 512–539.
- Fritzsch, B. Evolution of the vestibulo-ocular system. Otolaryngol. Head Neck Surg. 1998, 119, 182–192.
- Fritzsch, B.; Signore, M.; Simeone, A. Otx1 null mutant mice show partial segregation of sensory epithelia comparable to lamprey ears. Dev. Genes Evol. 2001, 211, 388–396.
- Fritzsch, B.; Wake, M. The inner ear of gymnophione amphibians and its nerve supply: A comparative study of regressive events in a complex sensory system (Amphibia, Gymnophiona). Zoomorphology 1988, 108, 201–217.
- Fritzsch, B.; Pan, N.; Jahan, I.; Duncan, J.S.; Kopecky, B.J.; Elliott, K.L.; Kersigo, J.; Yang, T. Evolution and development of the tetrapod auditory system: An organ of Corti-centric perspective. Evol. Dev. 2013, 15, 63–79.
- Maklad, A.; Fritzsch, B. Development of vestibular afferent projections into the hindbrain and their central targets. Brain Res. Bull. 2003, 60, 497–510.
- Zecca, A.; Dyballa, S.; Voltes, A.; Bradley, R.; Pujades, C. The order and place of neuronal differentiation establish the topography of sensory projections and the entry points within the hindbrain. J. Neurosci. 2015, 35, 7475–7486.
- Matei, V.; Pauley, S.; Kaing, S.; Rowitch, D.; Beisel, K.; Morris, K.; Feng, F.; Jones, K.; Lee, J.; Fritzsch, B. Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell cycle exit. Dev. Dyn. 2005, 234, 633–650.
- Rubel, E.W.; Fritzsch, B. Auditory system development: Primary auditory neurons and their targets. Annu. Rev. Neurosci. 2002, 25, 51–101.
- Ruben, R.J. Development of the inner ear of the mouse: A radioautographic study of terminal mitoses. Acta Otolaryngol. 1967, 220, 1–44.
- De No, R.L. The Primary Acoustic Nuclei; Raven Press: New York, NY, USA, 1981.
- Schmidt, H.; Fritzsch, B. Npr2 null mutants show initial overshooting followed by reduction of spiral ganglion axon projections combined with near-normal cochleotopic projection. Cell Tissue Res. 2019, 378, 15–32.
- Elliott, K.L.; Kersigo, J.; Pan, N.; Jahan, I.; Fritzsch, B. Spiral Ganglion Neuron Projection Development to the Hindbrain in Mice Lacking Peripheral and/or Central Target Differentiation. Front. Neural Circuits 2017, 11, 25.
- Fritzsch, B.; Matei, V.; Nichols, D.; Bermingham, N.; Jones, K.; Beisel, K.; Wang, V. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 233, 570–583.
- Milinkeviciute, G.; Cramer, K.S. Development of the ascending auditory pathway. In The Senses; Fritzsch, B., Grothe, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, pp. 337–353.
- Duncan, J.S.; Fritzsch, B.; Houston, D.W.; Ketchum, E.M.; Kersigo, J.; Deans, M.R.; Elliott, K.L. Topologically correct central projections of tetrapod inner ear afferents require Fzd3. Sci. Rep. 2019, 9, 10298.
- Maklad, A.; Kamel, S.; Wong, E.; Fritzsch, B. Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell Tissue Res. 2010, 340, 303–321.
- Bell, C.C.; Maler, L. Central neuroanatomy of electrosensory systems in fish. In Electroreception; Springer: Berlin/Heidelberg, Germany, 2005; pp. 68–111.
- Millimaki, B.B.; Sweet, E.M.; Dhason, M.S.; Riley, B.B. Zebrafish atoh1 genes: Classic proneural activity in the inner ear and regulation by Fgf and Notch. Development 2007, 134, 295–305.
- Northcutt, R.G. Distribution and innervation of lateral line organs in the axolotl. J. Comp. Neurol. 1992, 325, 95–123.
- Pan, N.; Jahan, I.; Kersigo, J.; Duncan, J.S.; Kopecky, B.; Fritzsch, B. A novel Atoh1 “self-terminating” mouse model reveals the necessity of proper Atoh1 level and duration for hair cell differentiation and viability. PLoS ONE 2012, 7, e30358.
- Xiang, M.; Maklad, A.; Pirvola, U.; Fritzsch, B. Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC Neurosci. 2003, 4, 2.
This entry is offline, you can click here to edit this entry!
