Clavulanic acid is an irreversible β-lactamase enzyme inhibitor with a weak antibacterial activity produced by the filamentous actinomycete Streptomyces clavuligerus (S. clavuligerus) and, in a lesser extent, by other streptomyces species. Clavulanic acid is typically co-formulated with broad-spectrum β‑lactam antibiotics such as amoxicillin and ticarcillin, conferring them high potential to treat infectious diseases caused by β‑lactam-resistant bacteria like Escherichia coli, Staphylococcus aureus, Neisseria gonorrhoeae and Streptococcus pneumonia.
- clavulanic acid
- Streptomyces clavuligerus
- systems biology
- strain engineering
- downstream pro-cessing.
1. Introduction
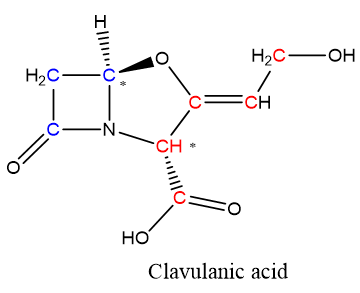
Clavulanic acid is a β-lactam compound with modest antibiotic activity but high inhibition capacity of β-lactamase enzymes [1]. The clavulanic acid molecule is an analog of the penicillin nucleus, in which the characteristic sulfur atom has been substituted by an oxygen atom. Clavulanic acid is one of the so-called “clavam metabolites” produced by the filamentous bacterium Streptomyces clavuligerus (S. clavuligerus); most of those metabolites have the characteristic fused bicyclic β-lactam/oxazolidine ring. Nevertheless, the clavulanic acid molecule (Figure 1) has 3R, 5R stereochemistry, opposite to the 3S, 5S configuration present in other clavam metabolites, which do not exhibit β-lactamase inhibition activity, although some of them have antibacterial or antifungal properties [2]. In addition to the stereochemistry, the inhibitory effect of clavulanic acid has been explained by the presence of the β-lactam/oxazolidine ring that bonds irreversibly with a serine residue in the catalytic center of the β-lactamase enzyme, thus rendering it inactive [3]. Currently, clavulanic acid is used in combination with other β-lactam antibiotics as an effective treatment against several clinical syndromes including pneumonia and exacerbations of chronic obstructive pulmonary disease, complicated intra-abdominal infections, acute infectious diarrhea, urinary tract infections, pharyngitis, surgical, wound, and skin infections [4]. Some of them are caused by resistant pathogenic bacteria already included in the World Health Organization priority list: Escherichia coli, Staphylococcus aureus, Neisseria gonorrhoeae, Streptococcus pneumonia, and all Enterobacteriaceae and Klebsiella species [5].
Figure 1. Clavulanic acid structure. Red and blue C atoms correspond to those coming from C-3 and C-5 precursors, respectively. * Stereochemical centers on clavulanic acid structure.
The antibiotic resistance phenomenon emerged along with the antibiotic era [6][7]. Years before penicillin was used at global scale, a penicillinase enzyme able to inactivate penicillin was discovered in bacteria extracts [8]. Antibiotic resistance has forced humanity to maintain an endless search for new and more powerful antibiotics. In this regard, the pharmaceutical industry plays a key role in the development of effective treatments against such multidrug-resistant bacteria [5]. Since the discovery of benzylpenicillin in the 1920s, the class of compounds referred to as β-lactam antibiotics has been the most extensively used antibiotics. Nevertheless, a significant number of different antibiotic compounds (such as carbapenems, cephamycins, cephalosporins, and monobactams) has been developed and implemented in the clinical practice as a strategy to evade the acquired resistance [9]. The new combinations of antibiotics are aimed to increase their spectrum of activity and overcome the resistance barriers developed by the bacteria. In order to mitigate the bacterial resistance to β-lactam antibiotics, several compounds have been identified as β-lactamase inhibitors. Those compounds can irreversibly inactivate the β-lactamases allowing the β-lactam antibiotics to act against the infection. The main β-lactamase inhibitors are Sulbactam, Tazobactam, and clavulanic acid, commercialized as sodium or potassium clavulanate salt.
Clavulanic acid is produced worldwide at large scale by several pharmaceutical companies, and it is also prescribed in more than 150 countries [1]. Clavulanic acid has a relatively limited market availability and a middle–high cost for the health system, especially in the developing countries when compared with the income level, being quite inaccessible for people without health insurance. The cost of clavulanic acid is mainly related to the complexity of the production process, the current uncertainties about the regulatory elements controlling the clavulanic acid biosynthetic gene cluster and the intellectual property associated with its production [2]. Despite the significant number of studies related to clavulanic acid production in S. clavuligerus submerged cultivations, low titers (~1 g∙L-1) are still obtained when using a wild type strain. The productivity of clavulanic acid production bioprocess is also compromised by the downstream processing: clavulanic acid separation from fermentation broths and precipitation as clavulanate salt.
2. Clavulanic acid biosynthesis in S. clavuligerus
The Streptomyces genus produces a wide variety of secondary metabolites with antimicrobial activity (approximately two-thirds of which occur naturally) [10]. In 1971, Nagarajan et al. [11] reported a new Streptomycete species as producer of two cephalosporin compounds. This new species was then named and described as S. clavuligerus by Higgens and Kastner, also in 1971 [12]. Later, in 1976, Howarth and Brown [13] described the clavulanic acid chemical structure, which was elucidated via spectroscopic and X-ray analyses and reported as a novel fused β-lactam compound with a significant inhibitory activity of β-lactamases. In 1977, Reading and Cole described the cultivation conditions of S. clavuligerus to produce clavulanic acid and the spectrometric method for clavulanic acid detection [3].
In 1941, the biochemist Selman Waskman described the most accepted definition of “antibiotic” as a small molecule made by microorganisms that inhibits the growth of another microorganism. During the “golden age” of antibiotics, approximately 70–80% of the antibiotics discovered came from Streptomycetes, but the evolutionary reason for the development of antibiotic biosynthetic capacity of soil bacteria and its ecological role are still unknown. Soil bacteria are not very efficient at up-taking nutrients, and their growth rate is considerably low in comparison with other bacteria and fungi. A plausible hypothesis that is widely accepted implies that antibiotics secretion allows the producer to control the organisms competing for the same nutritional resources in a hostile multispecies environment [14]. This is consistent with the secondary nature of antibiotics secretion under nutritional restriction. However, the antibiotic compounds at very low concentrations can modulate the transcriptional profiles of target bacteria and the products of resistance genes would silence those messages, opposing the theory of the development of attack and defense mechanisms [14]. Mathematical estimations point out that Streptomycetes are able to produce around 100,000 antibiotics and only 3% of them have been discovered [15]. Similarly, the existence of cryptic biosynthetic gene clusters (BGCs) suggests a higher capacity of antibiotic production in those organisms [16][17][18].
Clavulanic acid is secreted as a secondary metabolite, also referred as a specialized metabolite, by S. clavuligerus under nutritional restriction. The role of clavulanic acid in the physiology and adaptation of S. clavuligerus is also unknown. As a β-lactamase inhibitor, clavulanic acid may be part of a self-resistance mechanism involving the synthesis of β-lactam antibiotics, β-lactamase enzymes, and β-lactamase inhibitors; possibly developed by Streptomyces species to defend themselves from the effect of the antimicrobials [10]. Clavulanic acid biosynthesis is induced during phosphate limited conditions; recently it has been proposed that clavulanic acid production may be a consequence of a homeostatic response aimed to compensate for the ATP deficit under phosphate depletion [19]. This mechanism would trigger a strong activation of oxidative and amino acid metabolism, producing reduced cofactors and ATP and favoring the antibiotic biosynthesis as a feasible mechanism to adjust the ATP generation [19]. Moreover, the simultaneous secretion of penicillin and cephalosporins antibiotics along with β-lactamase enzymes and β-lactamase inhibitors would tightly control the energetic impact of an ATP-consuming futile cycle of polymerization/degradation of the cell wall caused by the β-lactam antibiotics accumulation [19].
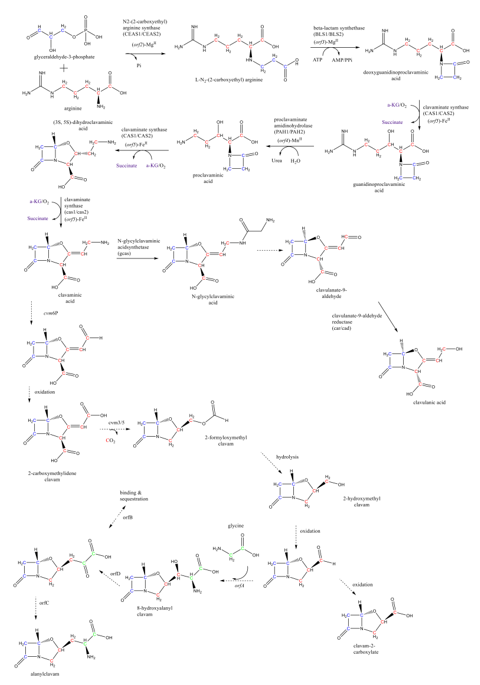
Clavulanic acid is a product of the clavam pathway, which proceeds towards two branches: the clavulanic acid and the clavams 5S biosynthesis [2]. The clavam pathway contains two sets of reactions: the so-called “early” and “late” steps. The early steps start with the condensation of the glycolysis intermediate glyceraldehyde-3-phosphate and the amino acid L-arginine, producing the N2-(2-carboxyethyl)-arginine. This condensation is catalyzed by the N2-(2-carboxyethyl)-arginine synthase (CEAS). The early steps comprise five well-known reactions leading to the (3S, 5S)-clavaminic acid. The formation of the clavaminic acid is considered a bifurcation point of the carbon flowing through the pathway. The clavulanic acid biosynthesis late steps consider the reactions leading to clavulanic acid, as well as the reactions forming the clavam 5S compounds after the clavaminic acid bifurcation [20]. The known reactions of the clavam pathway are summarized in Figure 2. The β-lactam compound deoxyguanidinoproclavaminic acid is synthesized from the N2-(2-carboxyethyl)-arginine in the second reaction of the clavam pathway, catalyzed by the β-lactam synthetase (BLS) [21][22]. The hydroxylation of the deoxyguanidinoproclavaminic acid takes place to form the guanidinoproclavaminic acid; a reaction mediated by clavaminate synthase (CAS) [22][23]. Subsequently, the proclavaminic acid is produced by removing the amidino group from the arginine residue on the guanidinoproclavaminic acid, by action of proclavaminate amidino hydrolase (PAH) [24]. The dihydroclavaminic acid is obtained from the proclavaminic acid through oxidative cyclization and desaturation, both catalyzed by CAS, leading to the (3S, 5S)-clavaminic acid [22][25]. At this point, the late steps start with the carbon flux bifurcation in two branches: one branch leading to the clavulanate-9-aldehyde and further the clavulanic acid, and the other branch forming clavam 5S compounds. It is important to highlight that the 3S, 5S stereochemistry of clavaminic acid is conserved in the synthesis of all clavam 5S compounds. In the case of the clavulanic acid, a transition from 3S, 5S configuration to 3R, 5R is required. Some authors have suggested that this stereochemical inversion may occur via the N-glycyl-clavaminic acid intermediate to form the clavulanate-9-aldehyde with a 3R, 5R configuration, and therefore the clavulanic acid [26][27]. However, more experimental evidence is required for the complete elucidation of intermediate reactions connecting the clavaminic acid with the clavulanate-9-aldehyde [26][27], which is lastly reduced to clavulanic acid by the action of clavulanate dehydrogenase (CAD) [28].
In addition to the clavulanic acid, other metabolites have been identified as side compounds of this biosynthetic pathway, namely, N-glycyl-clavaminic acid, N-acetylglycyl-clavaminic acid, and N-acetyl-clavaminic acid. It has been suggested that those compounds result from the intermediate steps involved in the transition of the 3S, 5S stereochemistry into the 3R, 5R of clavulanic acid [26]. Additionally, several metabolites (2-hydroxymethylclavam, 2-formyloxymethylclavam, clavam-2-carboxylic acid, and alanylclavam) have been identified and grouped as clavam 5S compounds due to their 3S, 5S stereochemistry [27][29][30]. Despite their structural similarity and common precursors, only the clavam compounds with a bicyclic nucleus formed by the β-lactam ring and an oxazolidine ring with 3R, 5R stereochemistry can effectively inhibit the β-lactamases [29].
Glycerol is the main substrate in the clavulanic acid production by S. clavuligerus as it has direct incorporation into the glycolytic pathway by forming glyceraldehyde 3-phosphate, the first C-3 precursor of clavulanic acid [31][32]. Then, the carbon flux splits into three pathways, two of them belonging to the primary metabolism: glycolysis and gluconeogenesis, and one belonging to the secondary metabolism: the clavam pathway [32]. In addition to glyceraldehyde 3-phosphate, the need for a C-5 precursor implies the constant demand for L-arginine. This amino acid is synthesized in the urea cycle while L-glutamate and L-aspartate promote its biosynthesis by fueling the urea cycle in the oxidative direction [19][32].
Figure 2. Summary of the CA biosynthetic pathway in S. clavuligerus. The green, blue, and red Cs represent the carbon atoms coming from metabolic intermediates such as glycine, glyceraldehyde 3-phosphate, and L-arginine, respectively. Adapted from the work in [43].
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics10010084
References
- Howard Ramirez-Malule; Bibliometric Analysis of Global Research on Clavulanic Acid. Antibiotics 2018, 7, 102, 10.3390/antibiotics7040102.
- Parag S. Saudagar; Shrikant A. Survase; Rekha S. Singhal; Clavulanic acid: A review. Biotechnology Advances 2008, 26, 335-351, 10.1016/j.biotechadv.2008.03.002.
- C. Reading; M. Cole; Clavulanic Acid: a Beta-Lactamase-Inhibiting Beta-Lactam from Streptomyces clavuligerus. Antimicrobial Agents and Chemotherapy 1977, 11, 852-857, 10.1128/aac.11.5.852.
- A. Huttner; J. Bielicki; M.N. Clements; N. Frimodt-Møller; A.E. Muller; J.-P. Paccaud; J.W. Mouton; Oral amoxicillin and amoxicillin–clavulanic acid: properties, indications and usage. Clinical Microbiology and Infection 2019, 26, 871-879, 10.1016/j.cmi.2019.11.028.
- David Gómez-Ríos1; Howard Ramírez-Malule2; Bibliometric analysis of recent research on multidrug and antibiotics resistance (2017–2018). Journal of Applied Pharmaceutical Science 2019, 9, 112-116, 10.7324/japs.2019.90515.
- Hannah Landecker; Antibiotic Resistance and the Biology of History. Body & Society 2016, 22, 19-52, 10.1177/1357034x14561341.
- Julian Davies; Dorothy Davies; Origins and Evolution of Antibiotic Resistance. Microbiology and Molecular Biology Reviews 2010, 74, 417-433, 10.1128/mmbr.00016-10.
- E. P. Abraham; E. Chain; An Enzyme from Bacteria able to Destroy Penicillin. Nature 1940, 146, 837-837, 10.1038/146837a0.
- Karen Bush; Patricia Bradford; β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harbor Perspectives in Medicine 2016, 6, a025247, 10.1101/cshperspect.a025247.
- Daniela De Araújo Viana Marques; Suellen Emilliany Feitosa Machado; Valéria Carvalho Santos Ebinuma; Carolina De Albuquerque Lima Duarte; Attilio Converti; Ana Lúcia Figueiredo Porto; Production of β-Lactamase Inhibitors by Streptomyces Species. Antibiotics 2018, 7, 61, 10.3390/antibiotics7030061.
- Ramanathan Nagarajan; Laverne D. Boeck; Marvin Gorman; Robert L. Hamill; Calvin E. Higgens; Marvin M. Hoehn; William M. Stark; Joel G. Whitney; .beta.-Lactam antibiotics from Streptomyces. Journal of the American Chemical Society 1971, 93, 2308-2310, 10.1021/ja00738a035.
- C. E. Higgens; R. E. Kastner; Streptomyces clavuligerus sp. nov., a -Lactam Antibiotic Producer. International Bulletin of Bacteriological Nomenclature and Taxonomy 1971, 21, 326-331, 10.1099/00207713-21-4-326.
- T. Trefor Howarth; Allan G. Brown; Trevor J. King; Clavulanic acid, a novel β-lactam isolated from Streptomyces clavuligerus; X-ray crystal structure analysis. J. Chem. Soc., Chem. Commun. 1975, 0, 266b-267, 10.1039/c3976000266b.
- Jon Clardy; Michael Fischbach; Cameron R. Currie; The natural history of antibiotics. Current Biology 2009, 19, R437-R441, 10.1016/j.cub.2009.04.001.
- Milind G. Watve; Rashmi Tickoo; Maithili M. Jog; Bhalachandra D. Bhole; How many antibiotics are produced by the genus Streptomyces ?. Archives of Microbiology 2001, 176, 386-390, 10.1007/s002030100345.
- Jingjing Xu; Jihui Zhang; Jiming Zhuo; Yue Li; Yuqing Tian; Huarong Tan; Activation and mechanism of a cryptic oviedomycin gene cluster via the disruption of a global regulatory gene, adpA, in Streptomyces ansochromogenes. Journal of Biological Chemistry 2017, 292, 19708-19720, 10.1074/jbc.m117.809145.
- Qinying Peng; Guixi Gao; Jin Lü; Qingshan Long; Xuefei Chen; Fei Zhang; Min Xu; Kai Liu; Yemin Wang; Zixin Deng; et al. Engineered Streptomyces lividans Strains for Optimal Identification and Expression of Cryptic Biosynthetic Gene Clusters. Frontiers in Microbiology 2018, 9, 3042, 10.3389/fmicb.2018.03042.
- Hue Thi Nguyen; Van Thuy Thi Pham; Chung Thanh Nguyen; Anaya Raj Pokhrel; Tae-Su Kim; Dahye Kim; Kun Na; Tokutaro Yamaguchi; Jae Kyung Sohng; Exploration of cryptic organic photosensitive compound as Zincphyrin IV in Streptomyces venezuelae ATCC 15439. Applied Microbiology and Biotechnology 2019, 104, 713-724, 10.1007/s00253-019-10262-x.
- David Gómez-Ríos; Victor A. López-Agudelo; Howard Ramírez-Malule; Peter Neubauer; Stefan Junne; Silvia Ochoa; Rigoberto Ríos-Estepa; A Genome-Scale Insight into the Effect of Shear Stress During the Fed-Batch Production of Clavulanic Acid by Streptomyces Clavuligerus. Microorganisms 2020, 8, 1255, 10.3390/microorganisms8091255.
- Susan E. Jensen; Biosynthesis of clavam metabolites. Journal of Industrial Microbiology and Biotechnology 2012, 39, 1407-1419, 10.1007/s10295-012-1191-0.
- Brian O. Bachmann; Craig A. Townsend; Kinetic Mechanism of the β-Lactam Synthetase of Streptomyces clavuligerus. Biochemistry 2000, 39, 11187-11193, 10.1021/bi000709i.
- Kapil Tahlan; Hyeon Ung Park; Annie Wong; Perrin Beatty; Susan Jensen; Two Sets of Paralogous Genes Encode the Enzymes Involved in the Early Stages of Clavulanic Acid and Clavam Metabolite Biosynthesis in Streptomyces clavuligerus. Antimicrobial Agents and Chemotherapy 2004, 48, 930-939, 10.1128/aac.48.3.930-939.2004.
- Robert W. Busby; Margaret D.-T. Chang; Jet Wimp; Craig A. Townsend; Expression and Purification of Two Isozymes of Clavaminate Synthase and Initial Characterization of the Iron Binding Site. Journal of Biological Chemistry 1995, 270, 4262-4269, 10.1074/jbc.270.9.4262.
- Matthew E. C. Caines; Jon Elkins; Kirsty S. Hewitson; Christopher J. Schofield; Crystal Structure and Mechanistic Implications of N2-(2-Carboxyethyl)arginine Synthase, the First Enzyme in the Clavulanic Acid Biosynthesis Pathway. Journal of Biological Chemistry 2004, 279, 5685-5692, 10.1074/jbc.m310803200.
- Biplav Shrestha; Dipesh Dhakal; Sumangala Darsandhari; Ramesh Pandey; Anaya Raj Pokhrel; Hum Nath Jnawali; Jae Kyung Sohng; Heterologous production of clavulanic acid intermediates in Streptomyces venezuelae. Biotechnology and Bioprocess Engineering 2017, 22, 359-365, 10.1007/s12257-017-0187-z.
- Howard Ramirez-Malule; Albeiro Restrepo; Wilson Cardona-Villada; Stefan Junne; Peter Neubauer; Rigoberto Rios-Estepa; Inversion of the stereochemical configuration (3S, 5S)-clavaminic acid into (3R, 5R)-clavulanic acid: A computationally-assisted approach based on experimental evidence. Journal of Theoretical Biology 2016, 395, 40-50, 10.1016/j.jtbi.2016.01.028.
- Haren Arulanantham; Nadia J. Kershaw; Kirsty S. Hewitson; Claire E. Hughes; Jan E. Thirkettle; Christopher J. Schofield; ORF17 from the Clavulanic Acid Biosynthesis Gene Cluster Catalyzes the ATP-dependent Formation of N-Glycyl-clavaminic Acid. Journal of Biological Chemistry 2005, 281, 279-287, 10.1074/jbc.m507711200.
- Alasdair K. MacKenzie; Nadia J. Kershaw; Helena Hernandez; Carol Robinson; Christopher J. Schofield; Inger Andersson; Clavulanic Acid Dehydrogenase: Structural and Biochemical Analysis of the Final Step in the Biosynthesis of the β-Lactamase Inhibitor Clavulanic Acid†,‡. Biochemistry 2007, 46, 1523-1533, 10.1021/bi061978x.
- Nathan J. Zelyas; Hui Cai; Thomas Kwong; Susan E. Jensen; Alanylclavam Biosynthetic Genes Are Clustered Together with One Group of Clavulanic Acid Biosynthetic Genes in Streptomyces clavuligerus. Journal of Bacteriology 2008, 190, 7957-7965, 10.1128/jb.00698-08.
- Sarah Goomeshi Nobary; Susan E. Jensen; A comparison of the clavam biosynthetic gene clusters in Streptomyces antibioticus Tü1718 and Streptomyces clavuligerus. Canadian Journal of Microbiology 2012, 58, 413-425, 10.1139/w2012-012.
- Michael E. Bushell; Samantha Kirk; Hong-Juan Zhao; Claudio A. Avignone-Rossa; Manipulation of the physiology of clavulanic acid biosynthesis with the aid of metabolic flux analysis. Enzyme and Microbial Technology 2006, 39, 149-157, 10.1016/j.enzmictec.2006.01.017.
- Howard Ramirez-Malule; Víctor López-Agudelo; David Gómez-Ríos; Silvia Ochoa; Rigoberto Ríos-Estepa; Stefan Junne; Peter Neubauer; TCA Cycle and Its Relationship with Clavulanic Acid Production: A Further Interpretation by Using a Reduced Genome-Scale Metabolic Model of Streptomyces clavuligerus. Bioengineering 2021, 8, 103, 10.3390/bioengineering8080103.