It sheds light on the triad "physical exercise-extracellular vesicles-depression" and suggests new avenues in this novel emerging field.
- depression
- physical exercise
- extracellular vesicles
1. Triad Physical Exercise-Extracellular Vesicles-Depression
Depression is associated with an increased risk of aging-related diseases. It is also seemingly a common psychological reaction to pandemic outbreaks with forced quarantines and lockdowns. Thus, depression represents, now more than ever, a major global health burden with therapeutic management challenges. Clinical data highlights that physical exercise is gaining momentum as a non-pharmacological intervention in depressive disorders. Although it may contribute to the reduction of systemic inflammation associated with depression, the mechanisms underlying the beneficial physical exercise effects in emotional behavior remain to be elucidated. Current investigations indicate that a rapid release of extracellular vesicles into the circulation might be the signaling mediators of systemic adaptations to physical exercise. These biological entities are now well-established intercellular communicators, playing a major role in relevant physiological and pathophysiological functions, including brain cell–cell communication. We also reviewed emerging evidence correlating depression with modified circulating extracellular vesicle surfaces and cargo signatures (e.g., microRNAs and proteins), envisioned as potential biomarkers for diagnosis, efficient disease stratification and appropriate therapeutic management.
2. Future Directions
Although the meta-analysis and guidelines strongly recognize PE as an effective therapeutic strategy for depressive disorders, the prescription of PE remains elusive. We believe that the lack of knowledge regarding its underlying neurobiological mechanisms may justify this scenario. This reinforces the need for more detailed investigation on the possible mediators. In fact, the present review aims to encourage and provide some guidance for scientific research towards that direction. Although the EV research field is relatively novel, some strong data is already emerging. Although the link between depressive disorders and altered circulating EV signature is the least supported, it is starting to be unraveled. In light of the current evidence, we still do not know if PE-triggered EVs contribute to the antidepressant effect of PE. However, the combination of the studies support our hypothesis (yellow circle illustrated in Figure 3), whereby circulating EVs contribute to the biological adaptations to PE in depression. Indeed, PE seems to change the concentration of circulating EVs and their cargo signatures (mostly protein and miRNA markers), possible mediators of vascular, metabolic, muscle, immune, inflammatory and neuronal adaptations during PE. Inflammation, particularly, seems to be a common mechanism in the PE-induced EV mechanism of action and in PE-induced positive effects in depression. Importantly, candidate biomarkers for depressive disorders often include inflammatory markers that are potentially carried by circulating EVs.
Finally, we encourage future studies designed to unveil the specific role of circulating EVs in tissue crosstalk, namely muscle-to-brain, and periphery-to-brain and brain-to-periphery signaling. Furthermore, we add additional candidate biomarkers that we consider as putative mediators of depression adaptations to PE (what else?, Figure 1). Those factors range from PE-induced exerkines (e.g., myostatin and, myonectin) to brain-derived protein markers (e.g., GFAP, α-synuclein (α-syn) and glutamine synthetase (GS)) and, also, include neurotrophic factors/receptors (e.g., BDNF, nerve growth factor (NGF), insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), tropomyosin receptor kinase A (TrkA) and tropomyosin receptor kinase B (TrkB)); signaling mediators of stress and inflammation (e.g., interleukins/cytokines, C reactive protein (CRP), corticotropin-releasing hormone (CRH), reactive oxygen species (ROS), superoxide dismutase (SOD), kynA, indoleamine 2,3-dioxygenase (IDO) and irisin); depression-associated miRNAs and proteins (e.g., aldolase C, reelin, tau and Aβ42); lysosomal proteases (e.g., cathepsin D); hormones involved in energy balance (e.g., leptin and ghrelin) and cholinergic mediators (e.g., acetylcholinesterase (AChE)). The assessment of those markers within isolated circulating EVs, in the context of both regular PE practice and depression, may provide a more comprehensive picture of the underlying signaling mechanisms. Ultimately, specific EV signatures could be translated into future diagnostic and/or therapeutic biomarkers. Finally, the uniqueness of EV miRNA and protein responses in certain physiologic (e.g., PE) or pathologic conditions (e.g., depression) strongly suggests that EVs must be further explored for a better understanding of their role in the body’s homeostasis.
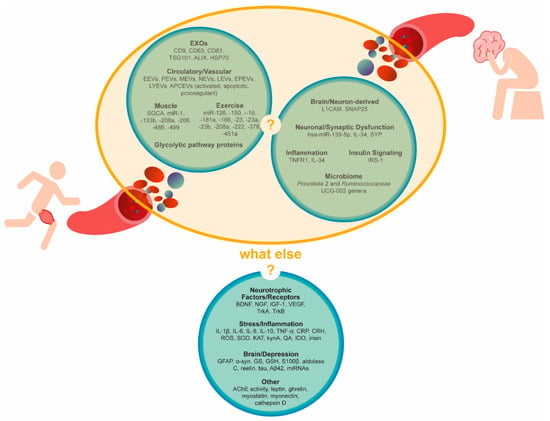
Figure 1. Circulating extracellular vesicle signatures after aerobic physical exercise or in the context of depression. Are they connected? Other candidate biomarkers. Circulating candidate biomarkers were suggested by the literature on physical exercise, inflammation or depression as either EV-cargoes or cell-free. AChE, acetylcholinesterase, APCEVs, antigen-presenting cell-derived vesicles, Aβ42, amyloid β42 BDNF, brain-derived neurotrophic factor, CRH, corticotropin-releasing hormone, CRP, C reactive protein, EEVs, endothelial-derived vesicles, EPEVs, endothelial progenitor cell-derived vesicles, EVs, extracellular vesicles, EXOs, exosomes, GFAP, glial fibrillary acidic protein, GS, glutamine synthetase, GSH, glutathione, HSP70, heat-shock protein 70, IDO, indoleamine 2,3-dioxygenase, IGF-1, insulin-like growth factor 1, IL, interleukin, IRS-1, insulin receptor substrate, KAT, kynurenine aminotransferase, kynA, kynurenic acid, LEVs, leucocyte-derived vesicles, LYEVs, lymphocyte-derived vesicles, L1CAM, L1 cell adhesion molecules, MEVs, monocyte-derived vesicles, NEVs, neutrophil-derived vesicles, NGF, nerve growth factor, PEVs, platelet-derived vesicles, QA, quinolinic acid, ROS, reactive oxygen species, S100β, S100 calcium-binding protein β, SNAP25, synaptosomal nerve-associated protein 25, SOD, superoxide dismutase, SYP, synaptophysin, TNF-α, tumor necrosis factor α, TrkA, tropomyosin receptor kinase A, TrkB, tropomyosin receptor kinase B, TSG101, tumor susceptibility 101, VEGF, vascular endothelial growth factor and α-syn, α-synuclein. Those factors range from PE-induced exerkines (e.g., myostatin and, myonectin) to brain-derived protein markers (e.g., GFAP, α-synuclein (α-syn) and glutamine synthetase (GS)) and, also, include neurotrophic factors/receptors (e.g., BDNF, nerve growth factor (NGF), insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), tropomyosin receptor kinase A (TrkA) and tropomyosin receptor kinase B (TrkB)); signaling mediators of stress and inflammation (e.g., interleukins/cytokines, C reactive protein (CRP), corticotropin-releasing hormone (CRH), reactive oxygen species (ROS), superoxide dismutase (SOD), kynA, indoleamine 2,3-dioxygenase (IDO) and irisin); depression-associated miRNAs and proteins (e.g., aldolase C, reelin, tau and Aβ42); lysosomal proteases (e.g., cathepsin D); hormones involved in energy balance (e.g., leptin and ghrelin) and cholinergic mediators (e.g., acetylcholinesterase (AChE)). The assessment of those markers within isolated circulating EVs, in the context of both regular PE practice and depression, may provide a more comprehensive picture of the underlying signaling mechanisms. Ultimately, specific EV signatures could be translated into future diagnostic and/or therapeutic biomarkers. Finally, the uniqueness of EV miRNA and protein responses in certain physiologic (e.g., PE) or pathologic conditions (e.g., depression) strongly suggests that EVs must be further explored for a better understanding of their role in the body’s homeostasis.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22020542
