Banana is a tropical fruit grown in more than 130 countries. It is the second most produced fruit after citrus, contributing around 16% of world fruit production and the fourth most important food crop after rice, wheat and corn. Banana is very nutritious and digests better than many other fruits. After harvest, almost 60% of banana biomass is left as waste. Worldwide, about 114.08 million metric tons of banana waste-loss are produced, leading to environmental problems such as the excessive emission of greenhouse gases. These wastes contain a high content of paramount industrial importance, such as cellulose, hemicellulose and natural fibers that various processes can modify, such as bacterial fermentation and anaerobic degradation, to obtain bioplastics, organic fertilizers and biofuels such as ethanol, biogas, hydrogen and biodiesel. In addition, they can be used in wastewater treatment methods by producing low-cost biofilters and obtaining activated carbon from rachis and banana peel. Furthermore, nanometric fibers commonly used in nanotechnology applications and silver nanoparticles useful in therapeutic cancer treatments, can be produced from banana pseudostems.
- banana waste-loss
- biofuels
- circular economy
- global banana production
- metabolite recovery
1. Introduction
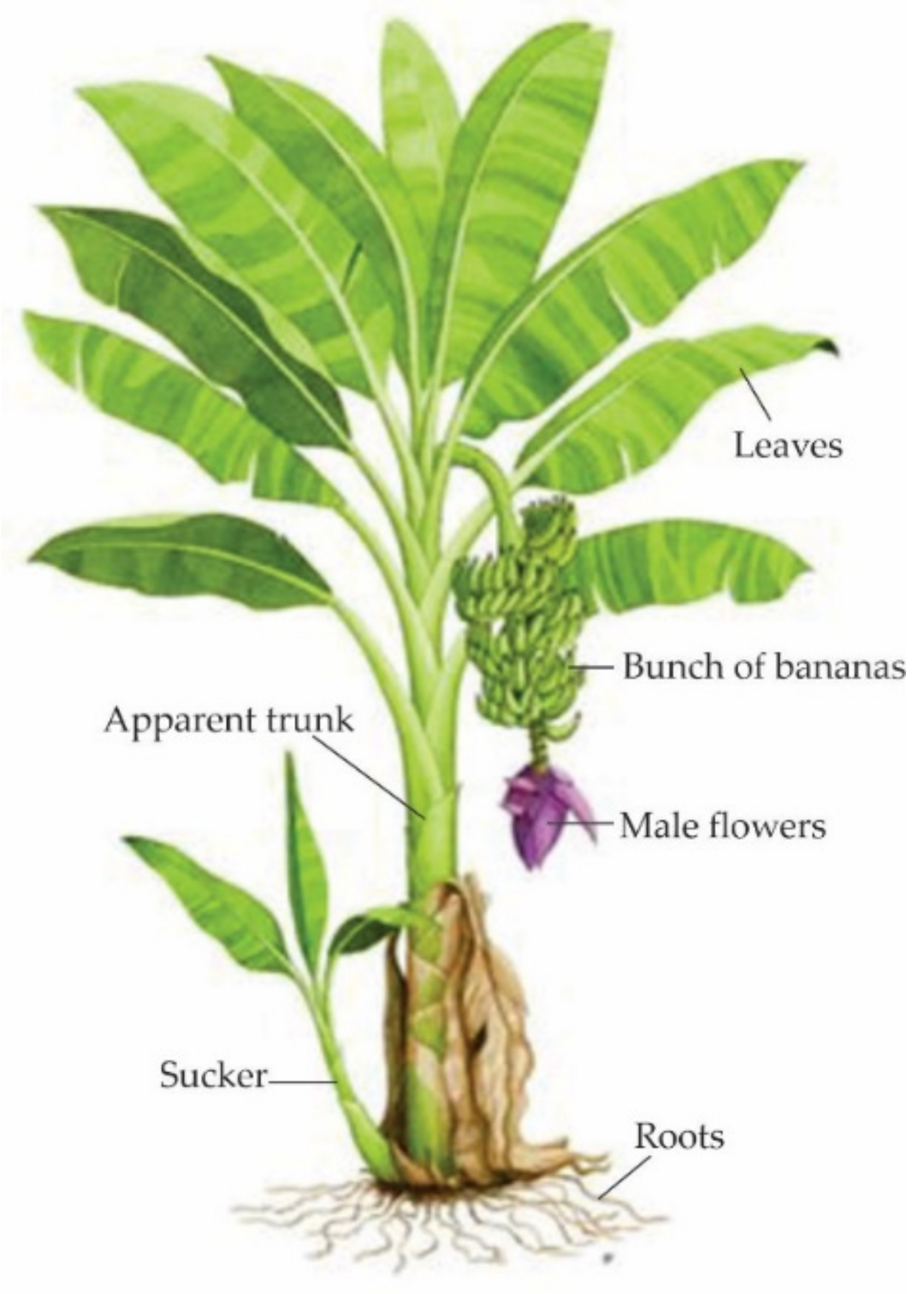
Figure 1. Parts of the banana plant. Adapted from [5].
| Producing Country | Variety | Genotype | Subgroup | References |
|---|---|---|---|---|
| Ecuador | Williams | AAA | Gros Michel | [6][7] |
| Dwarf Cavendish | AAA | Cavendish | ||
| Maqueño | AAB | Red | ||
| Gros Michel Highgate | AAA | Gros Michel | ||
| Philippines | Saba | ABB | Saba | [8][9] |
| Cavendish | AAA | Cavendish | ||
| Lakatan | AAA | Lakatan | ||
| Colombia | Banana Valery | AAA | Cavendish | [10] |
| Hartón Enano | AAB | Plantain | ||
| Bocadillo del Quindío | AA | Sucrier | ||
| Guineo Común | AAAae | Lujujira | ||
| India | Robusta | AAA | Cavendish | [11][12] |
| Palayankodan | AAB | Mysore | ||
| Nendran | AAB | Cavendish | ||
| Red banana | AAA | Red | ||
| Perú | Williams | AAA | Cavendish | [13] |
| FHIA-17 | AAAA | Non-defined | ||
| Cavendish Valery | AAA | Cavendish |
Chemical Composition of Banana
| Compound | Content | Unit | References |
|---|---|---|---|
| Carbohydrates | 22–88 | g/100g DW | [15][22][23] |
| Dietary fiber | 2–5 | g/100g DW | [17][23][24] |
| Protein | 1–2 | g/100g DW | [15][25][26] |
| Grease | 0.3–1.78 | g/100g DW | [24][26][27] |
| AC | 5 | mg/100g FW | [17][23][25] |
| P | 350–485 | mg/100g FW | [17][18][23] |
| Mg | 26–27 | mg/100g FW | [15][17][23] |
| Vitamin C | 12.7 | mg/100g FW | [15][23][25] |
| Vitamin A | 12.4 | mg/100g *RAE | [23][25] |
| Folate | 20 | µg/100g FW | [15][23][25] |
| Cellulose | 5.47 | g/100g DW | [24][26] |
| Hemicellulose | 18.83 | g/100g DW | [16][26] |
| Serotonin | 28 | µg/g DW | [28][18] |
| Phenols | 1–8 | g/100g DW | [18][22][26] |
| Dopamine | 7.9–9.9 | µg/g DW | [28][29] |
| Putrescine | 25–50 | mg/kg DW | [20][30] |
| Norepinephrine | 1.9 | µg/g DW | [28] |
2. Use of Banana Waste-Loss
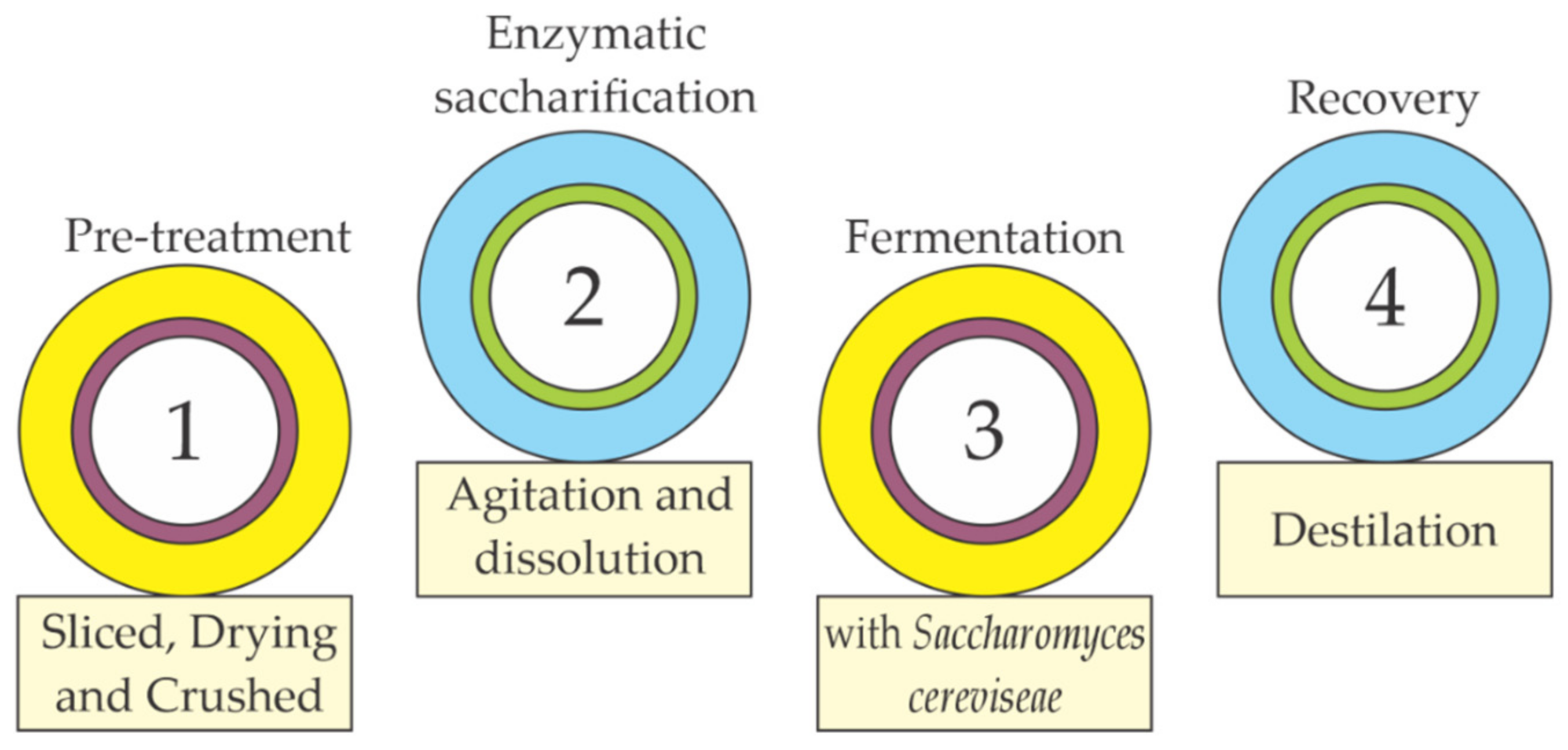
2.1. Biofuel Production
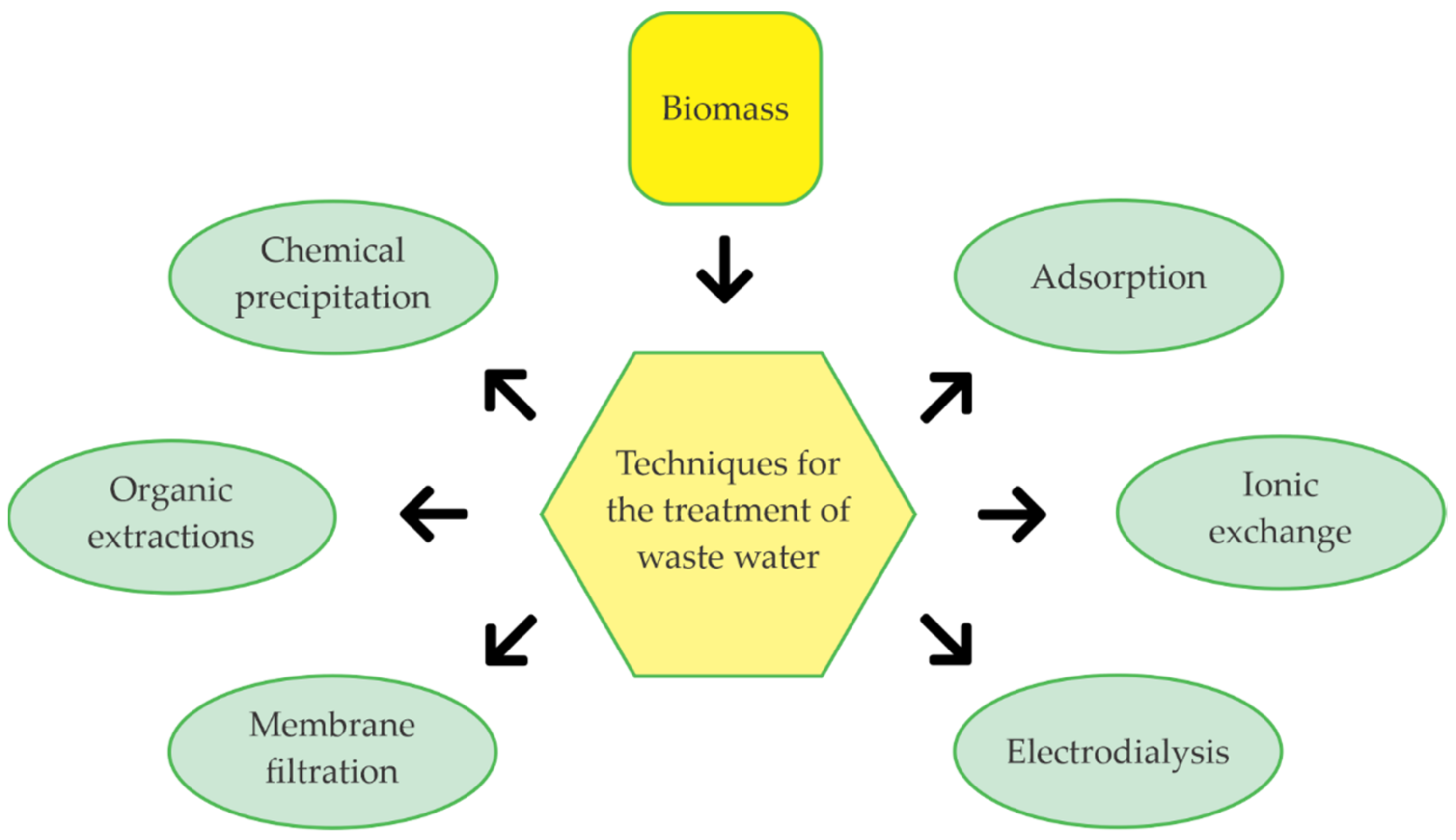
2.2. Wastewater Treatment
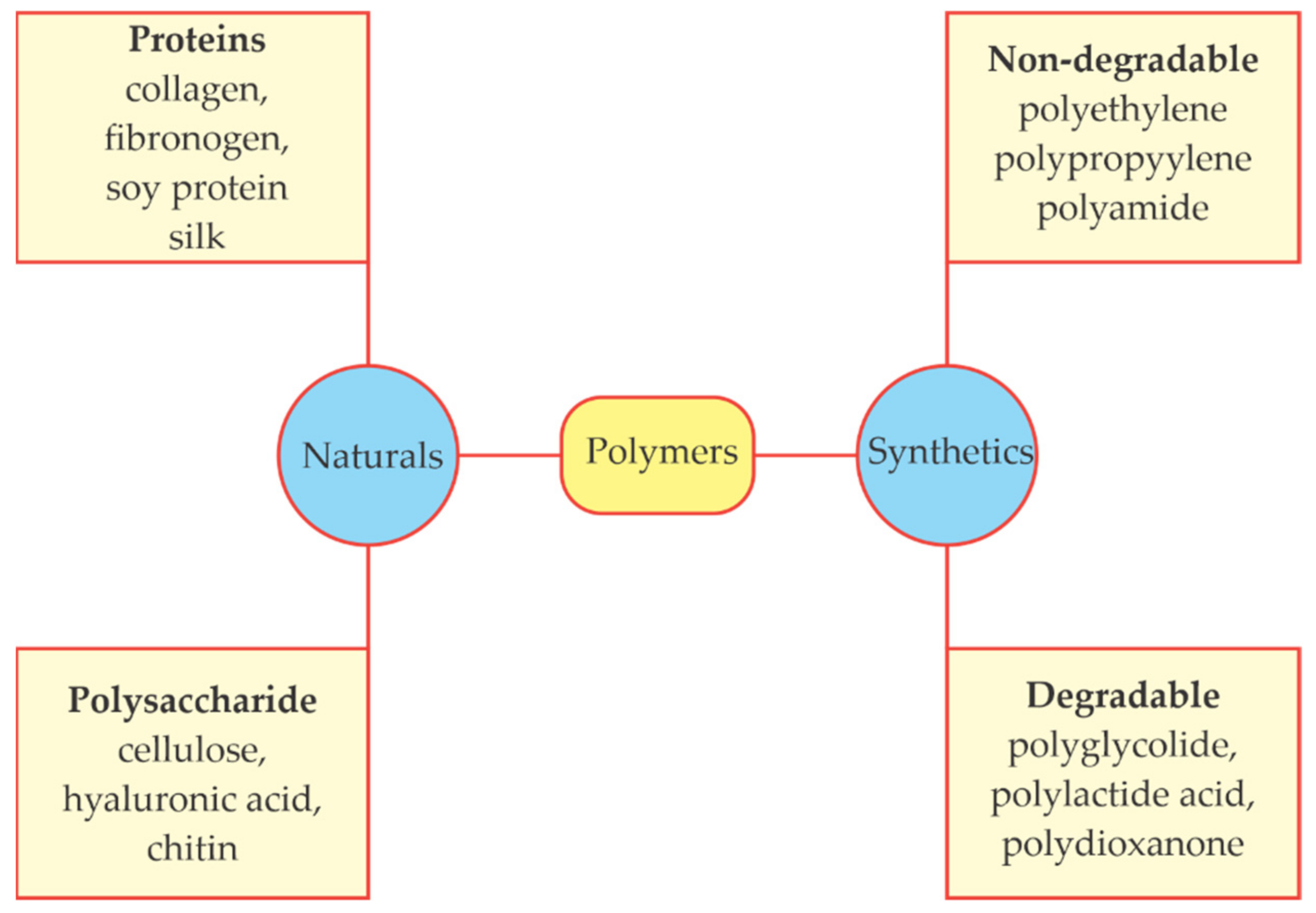
2.3. Bioplastics
2.4. Nanotechnology
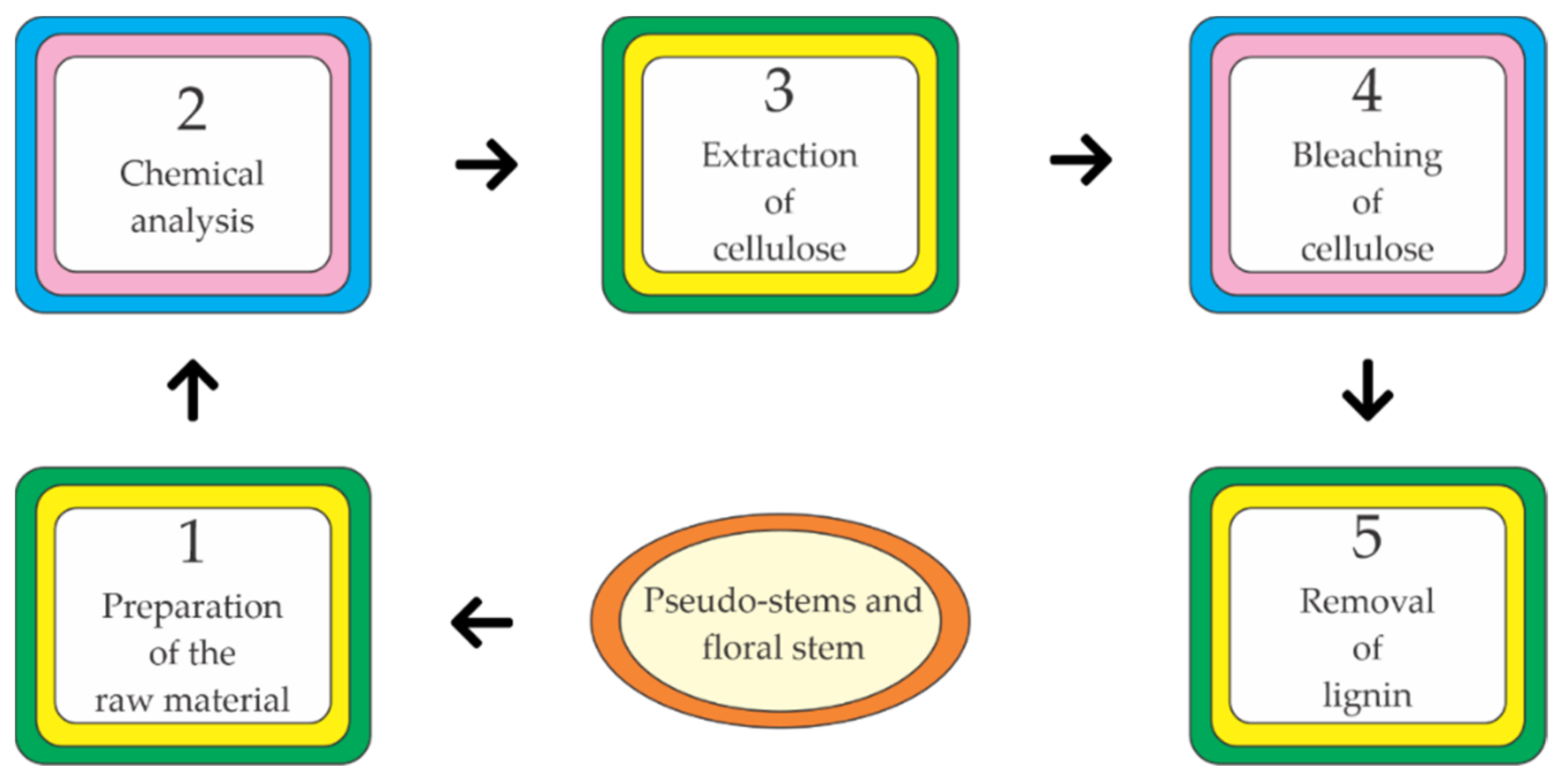
2.5. Organic Fertilizers
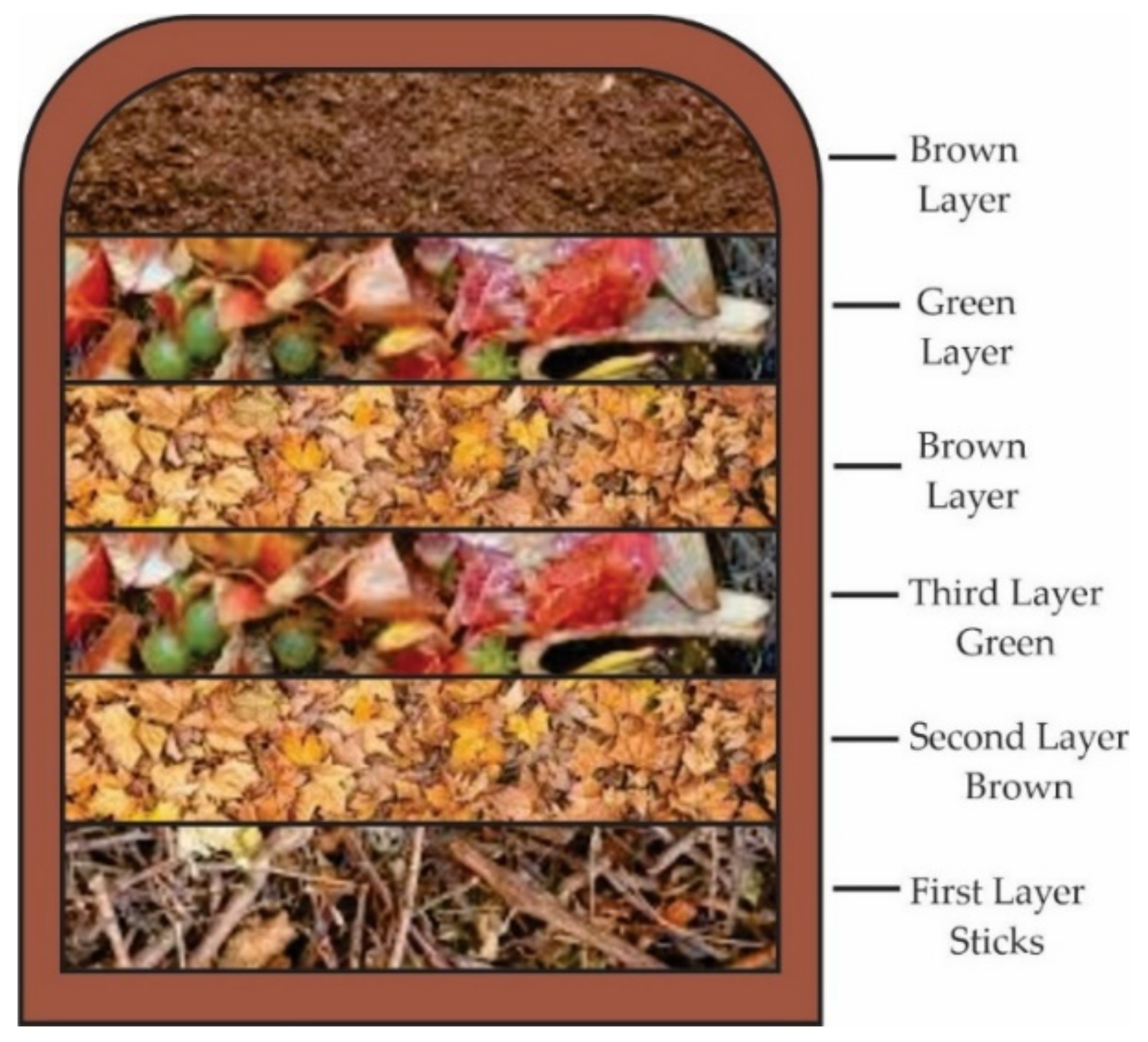
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/molecules26175282
References
- Amini, A.; Birch, J.; Bekhit, A. Production, application and health effects of banana pulp and peel flour in the food industry. J. Food Sci. Technol. 2019, 56, 548–559.
- Ahmad, T.; Danish, M. Prospects of banana waste utilization in wastewater treatment: A review. J. Environ. Manag. 2018, 206, 330–348.
- Tapia, C.; Paredes, N.; Lima, L. Diversity representativeness of the genus musa in Ecuador. ECUADOR ES CALIDAD 2019, 6, 53–58.
- Aguirre, J.; Castaño, V. Starch rheological characterization and morphological evaluation of 20 varieties of Musaceae (Musa sp.), from Fedeplatano Germplasm Bank, Chinchiná—Caldas, Colombia. Acta Agron. 2016, 65, 218–225.
- Diagram of Banana Plant [CCO]. Available online: https://www.flickr.com/photos/iita-media-library/5828333849/in/photostream/ (accessed on 18 July 2021).
- Amarasinghe, N.; Thilakarathna, G.; Wickramasinghe, I.; Wijesekara, I.; Deyalage, S. Functional, Physicochemical and antioxidant properties of flour and cookies fromtwo different banana varieties. Int. J. Food Sci. 2021, 2021, 1–9.
- Torres, L.; Zamora, L. Benefits in Latin America and the Caribbean about production of Cavendish AAA banana resistant to black Sigatoka. Rev. Bionatura 2018, 3, 740–744.
- Salvacion, A. Effect of climate on provincial-level banana yield in the Philippines. Inf. Process. Agric. 2020, 7, 50–57.
- Aguilar-Hawod, K.; Cumagun, C. Genetic diversity of Fusarium oxysporum f. sp. cubense causing Panama wilt of banana in the Philippines. Pathogens 2020, 9, 32.
- Lucas, J.; Velásquez, J.; Quintero, V. Evaluation of the thermal properties and composition starch extracted from 26 varieties of musa. Vitae 2016, 1, 551–556.
- Siji, S.; Nandini, P. Chemical and Nutrient Composition of Selected Banana Varieties of Kerala. Int. J. Adv. Eng. Manag. Sci. 2017, 3, 401–404.
- Kookal, S.; Thimmaiah, A. Nutritional Composition of Staple Food Bananas of Three Cultivars in India. Am. J. Plant. Sci. 2018, 9, 2480–2493.
- Escobar, H.; Andrade, J. Preliminary survey, diversity and population density of mites in banana, Musa AAA (Cavendish subgroup) cv. Williams in Peru. Int. J. Acarol. 2021, 47, 170–173.
- Laeliocattleya, R.A.; Estiasih, T.; Griselda, G.; Muchlisyiyah, J. The bioactive compounds and antioxidant activity of ethanol and ethyl ecetate extracts of Candi Banana (Musa paradisiaca). IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 012013.
- Elayabalan, S.; Subramaniam, S.; Shobana, V.; Ashok Kumar, K. An Overview on Phytochemical Composition of Banana (Musa spp.). Int. Bimon. Indian J. Nat. Sci. 2017, 7, 12408–12419.
- Rinah, N.; Adewale, O.; Tonna, A.; Jideani, A. Banana Bioactives: Absorption, Utilisation and Health Benefits. Intech 2019, 32, 137–144.
- Oyeyinka, B.; Afolayan, A. Comparative evaluation of the nutritive, mineral and antinutritive composition of Musa sinensis L. (banana) and Musa paradisiaca L. (plantain) fruit compartments. Plants 2019, 8, 598.
- Qamar, S.; Shaikh, A. Therapeutic potentials and compositional changes of valuable compounds from banana—A review. Trends Food Sci. Technol. 2018, 79, 1–9.
- Quiceno, M.; Giraldo, G.; Villamizar, R. Physical-chemical characterization of plantain (Musa paradisiaca sp. AAB, Simmonds) for industrialization. UGCiencia 2014, 20, 48–54.
- Sanlier, N.; Bektesoglu, M. Migraine and Biogenic Amines. Ann. Med. Health Sci. Res. 2021, 1362–1371.
- Medina, S.; Collado, J.; Ferreres, F.; Londoño, J.; Jiménez, C.; Guy, A.; Durand, T.; Galano, J.-M.; Gil, A. Valorization Strategy of Banana Passion Fruit Shell Wastes: An Innovative Source of Phytoprostanes and Phenolic Compounds and Their Potential Use in Pharmaceutical and Cosmetic Industries. J. Food Nutr. Res. 2017, 5, 801–808.
- Bakar, S.; Ahmad, N.; Jailani, F. Chemical and Functional Properties of Local Banana Peel Flour. J. Food Nutr. Res. 2018, 6, 492–496.
- Pareek, S. Nutritional and biochemical composition of banana (Musa spp.) cultivars. Nutr. Compos. Fruit Cultiv. 2016, 9, 49–81.
- Falcomer, A.; Riquette, R.; De Lima, B.; Ginani, V.; Zandonadi, R. Health benefits of green banana consumption: A systematic review. Nutrients 2019, 11, 1222.
- Pyar, H.; Peh, K. Chemical compositions of banana peels (Musa sapientum) fruits cultivated in Malaysia using proximate analysis. Res. J. Chem. Environ. 2018, 22, 108–113.
- Yesmin, B.; Sankar, D. Chemical profiling and functional properties of dietary fibre rich inner and outer bracts of culinary banana flower. J. Food Sci. Technol. 2019, 56, 5298–5308.
- Huang, S.; Martinez, M.; Bohrer, B. The compositional and functional attributes of commercial flours from tropical fruits (breadfruit and banana). Foods 2019, 8, 586.
- Sidhu, J.; Zafar, T. Bioactive compounds in banana fruits and their health benefits. Food Qual. Saf. 2018, 2, 183–188.
- Šeremet, D.; Durgo, K.; Jokić, S.; Hudek, A.; Cebin, A.; Mandura, A.; Jurasović, J.; Komes, D. Valorization of banana and red beetroot peels: Determination of basic macrocomponent composition, application of novel extraction methodology and assessment of biological activity in vitro. Sustainability 2020, 12, 4539.
- Sánchez, S.; Comas, O.; Rabell, J.; Veciana, T.; Latorre, L.; Vidal, C. Biogenic amines in plant-origin foods: Are they frequently underestimated in low-histamine diets? Foods 2018, 7, 205.
- Poveda, C.; Galeas, G. Evaluation of banana waste (masa acuminata cavendish subgroup) and cocoa (theobroma cacao), through the production of compost and biol. 3C Tecnol. Glosas Innov. Apl. Pyme 2020, 9, 17–29.
- Suárez, B.; Fernández, E.; Méndez, G.; Soto, D. Operational principles of circular economy for sustainable development: Linking theory and practice. J. Clean. Prod. 2019, 214, 952–961.
- Rada, E. Special waste valorization and renewable energy generation under a circular economy: Which priorities? WIT Trans. Ecol. Environ. 2019, 222, 145–157.
- Sharma, P.; Gaur, V.; Sirohi, R.; Varjani, S.; Hyoun, S.; Wong, J. Sustainable processing of food waste for production of bio-based products for circular bioeconomy. Bioresour. Technol. 2021, 325, 124684.
- Guerrero, A.; Ballesteros, I.; Ballesteros, M. The potential of agricultural banana waste for bioethanol production. Fuel 2018, 213, 176–185.
- Gumisiriza, R.; Hawumba, J.; Okure, M.; Hensel, O. Biomass waste-to-energy valorisation technologies: A review case for banana processing in Uganda. Biotechnol. Biofuels 2017, 10, 1–29.
- Zhang, Y.; Gao, Z.; Song, N.; Li, X. High-performance supercapacitors and batteries derived from activated banana-peel with porous structures. Electrochim. Acta 2016, 222, 1257–1266.
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.; Mohammadi, A. Different pretreatment technologies of lignocellulosic biomass for bioethanol production: An overview. Energy 2020, 199, 117457.
- Romero, H.; Macías, C.; Palacios, A.; Redrovan, F. Kinetic study of bioethanol production from agroindustrial residues of ripe banana peel. Ind. Data 2019, 22, 187–202.
- Adrianzen, F.P.C. Elaboración y Caracterización del Etanol a Partir de Residuos Industriales de Banano. Bachelor’s Thesis, Universidad Cesar Vallejo, Piura, Perú, 2018.
- Smuga, M.; Piskier, T.; Walendzik, B.; Szymanowska, D. Assessment of wasteland derived biomass for bioethanol production. Electron. J. Biotechnol. 2019, 41, 1–8.
- Uribe, R.; Smith, L.; Osorio, G.; Beatriz, B. Evaluation of activated carbon obtained from banana rachis in the purification of water intended for human consumption. Inst. Edwards Deming 2020, 1, 41–67.
- Pardo, S.; Karina, D.; Moncada, V. Evaluation of natural coagulants in water clarification. Rev. Investig. Agrar. Ambient. 2020, 11, 105–116.
- Valladares, M.; Valerio, C.; De la cruz, P.; Melgosa, R. Non-conventional absorbers: Sustainable alternatives for wastewater treatment. Rev. Ing. Univ. Medellín 2017, 16, 55–73.
- Davila, T.; Benitez, R.; Sanchez, N.; Ordoñes, D.; Muños, J. Evaluation of agroindustrial waste as bio-filters: Removal of cr (vi) in tannery synthetic effluents. Biotecnol. Sect. Agropecu. Agroind. 2017, 15, 49–58.
- Wang, B.; Sun, Y.; Sun, R. Fractionational and structural characterization of lignin and its modification as biosorbents for efficient removal of chromium from wastewater: A review. J. Leather Sci. Eng. 2019, 1, 1–25.
- Peng, H.; Guo, J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: A review. Environ. Chem. Lett. 2020, 18, 2055–2068.
- Antonieta, M.; Palma, R. Obtaining bioplastics from agricultural waste: A review of the Ecuador’s potentialities. Av. Quim. 2018, 13, 69–78.
- Antonio, F.; Auccahuasi, S.; Huamán, C.; Chipa, H.; Elizabeth, M.; Chacón, C. Biodegradability of bioplastics made from Mangifera indica and Musa paradisiaca peels. Cent. Agric. 2020, 47, 22–31.
- Jiménez, C. Evaluación de Polímeros en Pseudotallos de Musa acuminata AAA, Musa sapientum ABB y Musa paradisiaca AAB Para Elaboración de Bioplástico. Master’s Thesis, El Colegio de la Frontera Sur, San Cristóbal, Mexico, 2017.
- Manali, S.; Sanjukta, R.; Himanshu, P.; Archana, M. Bioplastic for future: A review then and now. World J. Adv. Res. Rev. 2021, 9, 056–067.
- Ekiert, M.; Mlyniec, A.; Uhl, T. The influence of degradation on the viscosity and molecular mass of poly(lactide acid) biopolymer. Diagnostyka 2015, 16, 63–70.
- Gómez, C.; Serpa, A.; Velásquez, J.; Gañán, P.; Castro, C.; Vélez, L.; Zuluaga, R. Vegetable nanocellulose in food science: A review. Food Hydrocoll. 2016, 57, 178–186.
- Hugo, R.; Saldivar, L.; Argüello, B.; Villarreal, G.; Reyes, V. Nanotechnology potential in sustainable agriculture. Acta Univ. 2018, 28, 9–24.
- Ávalos, A.; Haza, A.; Morales, P. Nanotechnology in the Food Industry. Rev. Complut. Cienc. Vet. 2016, 10, 1–17.
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 1–13.
- Naeem, M.; Siddiqui, Q.; Mushtaq, M.; Farooq, A.; Pang, Z.; Wei, Q. Insitu Self-Assembly of Bacterial Cellulose on Banana Fibers Extracted from Peels. J. Nat. Fibers 2020, 17, 1317–1328.
- Sudharmaidevi, C.R.; Thampatti, K.C.M.; Saifudeen, N. Rapid production of organic fertilizer from degradable waste by thermochemical processing. Int. J. Recycl. Org. Waste Agric. 2017, 6, 1–11.
- Ramírez, E. Management of local organic resources, as an agroecological strategy for the development of fertilizers, in cloud forests of the coastal mountain range Venezuela. Soil Sci. 2017, 45, 19–30.
- Ayilara, M.; Olanrewaju, O.; Babalola, O.; Odeyemi, O. Waste management through composting: Challenges and potentials. Sustainability 2020, 12, 4456.
- Lim, L.; Bong, C.; Lee, C.; Klemeš, J.; Sarmidi, M.; Lim, J. Review on the current composting practices and the potential of improvement using two-stage composting. Chem. Eng. Trans. 2017, 61, 1051–1056.
- El Barnossi, A.; Moussaid, F.; Iraqi, A. Tangerine, banana and pomegranate peels valorisation for sustainable environment: A review. Biotechnol. Reports 2021, 29, e00574.
- Guevara, C.; José, A.; Carlos, P. Biorefinery from banana rejected: Integrated system for ethanol, single cell protein, biogas and compost co-prod. Biotecnol. Sect. Agropecu. Agroind. 2016, 14, 78–86.
- Virk, A.; Memon, K.; Memon, M.; Hussain, S. Formulation of optimum banana residue based compost product and its efficacy on maize and soil properties. Indian J. Sci. Technol. 2021, 14, 932–941.
- Jędrczak, A. Composting and Fermentation of Biowaste—Advantages and Disadvantages of Processes. Civ. Environ. Eng. Rep. 2018, 28, 71–87.
- Yu, Z.; Tang, J.; Liao, H.; Liu, X.; Zhou, P.; Chen, Z.; Rensing, C.; Zhou, S. The distinctive microbial community improves composting efficiency in a full-scale hyperthermophilic composting plant. Bioresour. Technol. 2018, 265, 146–154.
- Candis, H.; Dana, W.; Melanie, P.; Matthew, G.; Michelle, K. Safe Community Gardening Practices: Focus Groups with Garden Leaders in Atlanta, Georgia. Physiol. Behav. 2020, 176, 139–148.
