Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nutrition & Dietetics
Epidemiological and experimental studies have suggested that diet is one of the environmental factors that contributes to the onset and pathophysiology of ulcerative colitis.
- diet
- inflammatory bowel disease
- ulcerative colitis
1. Introduction
Ulcerative colitis (UC)—a subtype of inflammatory bowel disease (IBD)—is a chronic, idiopathic inflammatory disease that affects the colon and is characterized by relapsing and remitting mucosal inflammation [1]. UC patients mostly present blood in the stool and diarrhea [1]. UC is associated with major morbidity in Western countries, and its incidence is increasing in developing countries [2]. The multifactorial pathophysiology of UC includes genetic predisposition, epithelial barrier defects, dysregulated immune responses, microbial dysbiosis, and environmental factors [1][2].
It has been suggested that environmental factors play a major role in the pathogenesis of IBD. Early-life events such as mode of birth, breastfeeding, and exposure to antibiotics and other factors such as air pollution, smoking, psychological state, exercise, and diet are among the potential environmental contributors of IBD development or disease activity [3].
Significant changes in dietary intake during the past decades have been associated with the increase in incidence of UC. The relationship between diet and UC development has been indicated in several epidemiological studies [4]. Two recent meta-analysis studies showed that soft drink consumption and sucrose intake were associated with 69% and 10% increased risk of UC development, respectively [5][6]. Consumption of fruits (odds ratio: 0.57) and vegetables (odds ratio: 0.71) was related to decreased odds of UC development in another meta-analysis study [7]. A significant association between meat intake (red meat in particular) and UC risk was found in a meta-analysis of seven epidemiological studies (summary relative risk: 1.47) [8]. Furthermore, whereas n-3 polyunsaturated fatty acids (PUFAs) content of diet was related to decreased odds of UC development (odds ratio: 0.56) [9], dietary arachidonic acid (an n-6 PUFA) as measured in adipose tissue increased risk of UC development (relative risk: 4.16) in a large prospective cohort study among Danish adults [10]. Although the exact pathophysiological mechanisms in which diet plays a role in IBD development remain unknown, several plausible explanations including its effects on composition of gut microbiota, production of microbial metabolites, alterations in mucosal immunity, and mucosal barrier function have been proposed [11] (Figure 1).
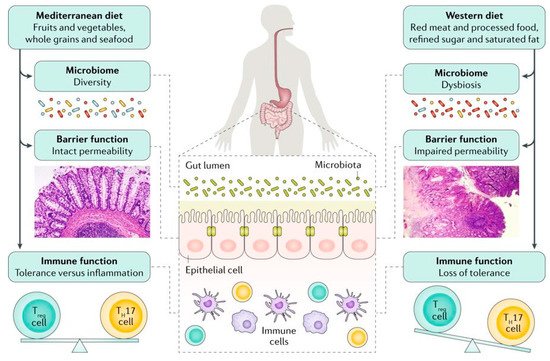
Figure 1. Although the exact mechanisms responsible for the association between diet and development of inflammatory bowel disease is unknown, several mechanisms have been suggested. An unhealthy dietary pattern such as a Western diet has been linked to changes in the gut microbiome and epithelial barrier function and seems to have a direct influence on immune function, triggering a pro-inflammatory environment characterized by an imbalance in the T helper 17 (TH17) cell to regulatory T (Treg) cell ratio [Adapted with permission [11]].
Abnormalities in the intestinal microbiota have been reported in some, but not all UC patients [1][12][13][14]. In some studies, UC patients have been shown to have decreased bacterial diversity, characterized by a decreased Firmicutes and increased Gammaproteobacteria and Enterobacteriaceae [15]. However, it is not clear if bacterial dysbiosis is the cause or effect of mucosal inflammation in UC [1][13] (Figure 2).
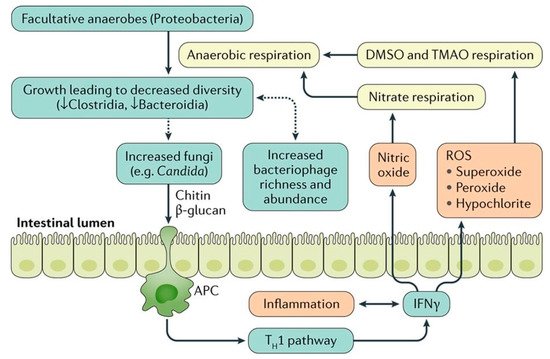
Figure 2. The relationship between gut microbiota and colonic inflammation in inflammatory bowel disease. Inflammation in colon stimulates production of Interferon gamma (IFN-γ) that eventually generates reactive oxygen species (ROS). ROS make products for anaerobic respiration. These products can be used by facultative anaerobes to outgrow, which leads to decreased bacterial diversity. The dysbiotic microbiota may further stimulate the growth of fungi that can worsen inflammation via chitin and β -glucan antigen-presenting cell (APC) activation of the type 1 T helper (TH1) pathway. In addition, the microbial dysbiosis is associated with increased bacteriophage richness and abundance, which can affect the bacterial microbiota via gene transfer. DMSO, dimethyl sulfoxide; TMAO, trimethylamine N-oxide. [Adapted with permission [13]].
Dietary factors can be related to UC pathogenesis or disease course through direct effects on the host or indirect effects through modulations of composition or function of gut microbiota. Diet has a major role in shaping gut microbial composition [16]. For instance, increased Bacteroidetes and decreased Firmicutes and Enterobacteriaceae in rural African children in comparison to European children were mainly attributed to differences in dietary patterns between the two populations [17]. Therefore, it has been suggested that diet-induced changes in microbiota may transform healthy gut microbiota into a disease-inducing entity that could either initiate or perpetuate inflammation in patients with IBD [16]. Agus et al. [18] indicated that a high fat/high sugar diet resulted in intestinal mucosal dysbiosis characterized by an overgrowth of pro-inflammatory proteobacteria and a decrease in protective bacteria. In addition, they showed that the transplantation of feces from high fat/high sugar fed mice to germ-free mice increased susceptibility to adherent-invasive Escherichia coli infection.
In addition to their significant effects on microbial composition, dietary factors can also affect the metabolic functions of gut microbiota. Short chain fatty acids (SCFA), which are defined as the groups of fatty acids with fewer than six carbons including formic acid (C1), acetic acid (C2), propionic acid (C3), butyric acid (C4), and valeric acid (C5), are derived from commensal bacterial fermentation of indigestible dietary fibers in both the small and large intestines [19][20]. Acetate, propionate and butyrate account for more than 95 % of all the SCFA content in the gut [19]. Acetate and butyrate in particular have an essential role in maintaining mucosal barrier function and modulating immune function [21][22] (Figure 3). SCFA regulate the functions of epithelial and/or immune cells through altering gene expression, cellular differentiation, chemotaxis, proliferation, and apoptosis [19]. The number of SCFA-producing bacteria such as Faecalibacterium prausnitzii is decreased in some UC patients, and these are inversely correlated with disease activity [23]. Moreover, a western diet characterized by high intake of sugar [18][24] and fat [18] and decreased amount of dietary fiber was associated with decreased SCFAs and increased susceptibility to colitis in experimental studies.
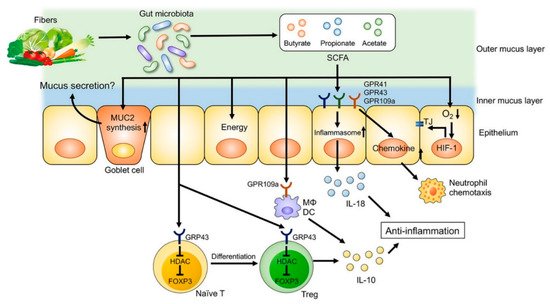
Figure 3. The role of fiber-derived short chain fatty acids (SCFAs) in regulation of intestinal homeostasis. SCFAs serve as energy substrates for colonocytes. In addition, SCFAs regulate intestinal barrier function and immune system through G-protein-coupled receptors (GPRs) signaling. SCFAs promote the differentiation of regulatory T (Treg) cells and the production of interleukin (IL)-10 through GPR43. Furthermore, SCFA facilitate inflammasome activation in colonic epithelial cells through GPR43, stimulating IL-18 production that is critical for anti-inflammation and epithelial repair. SCFAs also regulate intestinal barrier function via enhancing the expression of tight junction proteins and the synthesis of mucin (MUC)2. DC, dendritic cells; FOXP3, forkhead box P3; HDAC, histone deacetylases; Mϕ, macrophages; TJ, tight junctions. [Adapted with permission [22]].
2. Current Insights on Diet in Ulcerative Colitis
It has been suggested that environmental factors including diet play an important role in the pathophysiology of IBD and especially in UC, a chronic colonic inflammation. In the present article, after a brief overview of potential mechanisms in which diet plays a role in the pathogenesis of IBD, we then reviewed dietary intervention studies in UC patients.
The three randomized controlled trials that have been performed to assess the efficacy of dietary interventions for maintenance of remission in UC [25][26][27] were all focused on complete exclusion of one or more food items that were hypothesized to trigger IBD symptoms. Two of these studies [25][26] aimed to eliminate milk or dairy products; however, they failed to show a significant decrease in relapse rate in patients randomized to the elimination diet group in comparison to those randomized to the control diet. This finding is important as unnecessary dietary restrictions that lack supporting scientific evidence may result in several nutritional deficiencies (e.g., calcium due to exclusion of milk and dairy products) in IBD patients [28]. Therefore, patients should be informed by their health care team about the possible harmful effects of food elimination diets.
Here, the only elimination diet that was associated with a reduction in clinical relapse rate in UC patients who were in remission at baseline was a carrageenan-free diet [27]. Carrageenan belongs to a family of sulfated polysaccharides and are extracted from seaweeds. It is approved as “generally recognized as safe” by the United States Food and Drug Administration and is used in the food industry for its gelling, thickening, and stabilizing properties. It has been suggested that carrageenan may reduce protein and peptide bioaccessibility, disrupt normal epithelial function, and promote intestinal inflammation [29]. However, others have been skeptical about these findings, which are mainly derived from experimental animal studies [30]. The results from the randomized clinical trial in which a carrageenan-free diet was found to be related to lower relapse rate and decreased inflammation (as assessed by decreased serum interleukin-6 and FCP) should be interpreted with caution as the sample size of this multi-center trial was very small (n = 12) and the reported p-values obtained from parametric tests were marginally significant. Therefore, these interesting findings need to be confirmed in future well-powered randomized controlled studies.
We identified only two studies [31][26] that tested the efficacy of diet for induction of remission in UC patients. In the first study, exclusion of food items that were found to trigger UC-related symptoms was associated with higher clinical remission rate in comparison to a normal diet [31]. Although the elimination of foods was based on each participant’s self-reported food intolerance, there were some general recommendations regarding specific food groups/items such as dairy products, refined sugar, and beverages. However, the study was performed on a small number of patients (n = 18), and the duration of follow-up was short (6 weeks). In addition, the intervention did not result in endoscopic or histologic improvement in that time period. Furthermore, patients in the intervention group experienced a mean weight loss of 2.5 kg that was not explained in the study. The authors also reported that there was no food that triggered symptoms in all patients. However, spicy and curried foods and fruits (specially grapes, melon, and citruses) were commonly reported to provoke symptoms. In the second study, which was performed in pediatric UC patients with active disease, elimination of cow milk protein from diet was not beneficial neither for induction or for maintenance of remission during a one-year follow-up in comparison to a control diet [26]. As mentioned by the authors, the dietary restrictions that many IBD patients follow often are not supported by scientific evidence. These inappropriate diets reduce caloric intake and may contribute to malnutrition and micronutrient deficiencies, especially in pediatric patients. Whether a subgroup of patients with UC (e.g., patients with lactose intolerance or atopy) will benefit from elimination diets or not needs to be explored in future clinical trials.
In this review, we also included three other studies that recruited patients with active disease and UC remission concurrently. They reported the effectiveness of comprehensive dietary advices [32], low FODMAP [33] or IgG-guided exclusion diets [34] in reduction of disease activity in UC patients. Although these findings are encouraging, one of the major limitations of these studies is that they did not report their findings for patients with active disease versus patients in UC remission separately to allow meaningful interpretations [35]. Therefore, we suggest that in future studies the dietary interventions be focused on clearly specified groups of patients (e.g., active disease or in remission) or study outcomes to be reported for different groups of participants separately.
Diet is of major interest for IBD patients, and they use a variety of dietary strategies to manage their underlying disease and related symptoms [36]. Despite the significant role of diet in the development of IBD or management of gastrointestinal symptoms in these patients, we could identify only a few randomized controlled trials that assessed the efficacy of diet for induction of remission, maintenance of remission, or improvement of gastrointestinal symptoms in UC patients. In addition, in the previous studies, the underlying mechanisms in which diet may prevent increases in colonic or systemic inflammation and ultimately help patients to maintain remission have not been investigated. There are many omics fields involved in the study of pathogenesis of IBD such as genomics, metagenomics, transcriptomics, proteomics, and metabolomics [37]. As dietary factors have a significant impact on some of these key players of IBD development, investigating the changes in this multi-omic network of IBD during a controlled dietary intervention has the potential to elucidate the underlying mechanisms of diet-IBD interactions. High quality, well-powered human dietary intervention studies for management of IBD may include the following: Quantification of baseline habitual diet using appropriate tools such as food frequency questionnaires, monitoring of adherence to the diet using food recalls/records, large long-term controlled trials, use of a control diet to determine the specificity of observed effects to the intervention, use of a variety of subjective and objective endpoints (e.g., symptoms, quality of life, clinical biomarkers, endoscopic and histological evaluations) to monitor response to dietary interventions [36], and consider the use of omic-based assessments of serum, urine, stool, and/or intestinal biopsies to investigate underlying protective mechanisms. Considering findings from previous observational studies and clinical trials, investigating the potential benefits of following a healthy dietary pattern, such as experimental anti-inflammatory diets that incorporate several dietary recommendations, is of great value in the management of UC-related symptoms and inflammation. Furthermore, as indicated in elimination diet studies, food intolerances are individual-based, and not all patients will benefit from excluding certain food items/groups. Therefore, personalized dietary recommendations that take into account each patient’s food intolerances and food preferences should be the subject of future well-designed dietary trials in IBD patients.
3. Conclusions
Here, we found that there have been few well-designed and/or adequately powered randomized clinical trials to investigate the role of diet in maintenance of remission in UC patients. As suggested in a recent Cochrane systematic review [35], consensus on the composition of evidence-based dietary interventions in IBD patients is required and there is a need for more high-quality, well-powered, randomized, controlled trials to assess the efficacy of these interventions.
This entry is adapted from the peer-reviewed paper 10.3390/nu11071498
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770.
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165.
- Ananthakrishnan, A.N. Debate session: So what causes inflammatory bowel disease? It’s all in the environment. J. Gastroenterol. Hepatol. 2018, 33, 24.
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary Intake and Risk of Developing Inflammatory Bowel Disease: A Systematic Review of the Literature. Am. J. Gastroenterol. 2011, 106, 563–573.
- Nie, J.Y.; Zhao, Q. Beverage consumption and risk of ulcerative colitis: Systematic review and meta-analysis of epidemiological studies. Medicine (Baltimore) 2017, 96, e9070.
- Wang, F.; Feng, J.; Gao, Q.; Ma, M.; Lin, X.; Liu, J.; Li, J.; Zhao, Q. Carbohydrate and protein intake and risk of ulcerative colitis: Systematic review and dose-response meta-analysis of epidemiological studies. Clin. Nutr. 2017, 36, 1259–1265.
- Li, F.; Liu, X.; Wang, W.; Zhang, D. Consumption of vegetables and fruit and the risk of inflammatory bowel disease: a meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 623–630.
- Ge, J.; Han, T.-J.; Liu, J.; Li, J.-S.; Zhang, X.-H.; Wang, Y.; Li, Q.-Y.; Zhu, Q.; Yang, C.-M. Meat intake and risk of inflammatory bowel disease: A meta-analysis. Turk. J. Gastroenterol. 2015, 26, 492–497.
- John, S.; Luben, R.; Shrestha, S.S.; Welch, A.; Khaw, K.-T.; Hart, A.R. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: A UK prospective cohort study. Eur. J. Gastroenterol. Hepatol. 2010, 22, 602–606.
- De Silva, P.S.; Olsen, A.; Christensen, J.; Schmidt, E.B.; Overvaad, K.; Tjonneland, A.; Hart, A.R. An association between dietary arachidonic acid, measured in adipose tissue, and ulcerative colitis. Gastroenterology 2010, 139, 1912–1917.
- Khalili, H.; Chan, S.S.M.; Lochhead, P.; Ananthakrishnan, A.N.; Hart, A.R.; Chan, A.T. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 525–535.
- McIlroy, J.; Ianiro, G.; Mukhopadhya, I.; Hansen, R.; Hold, G.L. Review article: the gut microbiome in inflammatory bowel disease-avenues for microbial management. Aliment. Pharmacol. Ther. 2018, 47, 26–42.
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584.
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10.
- Frank, D.N.; Amand, A.L.S.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785.
- Brown, K.; DeCoffe, D.; Molcan, E.; Gibson, D.L. Diet-Induced Dysbiosis of the Intestinal Microbiota and the Effects on Immunity and Disease. Nutrients 2012, 4, 1095–1119.
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696.
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032.
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8.
- Neis, E.P.; van Eijk, H.M.; Lenaerts, K.; Olde Damink, S.W.; Blaak, E.E.; Dejong, C.H.; Rensen, S.S. Distal versus proximal intestinal short-chain fatty acid release in man. Gut 2019, 68, 764–765.
- Reddavide, R.; Rotolo, O.; Caruso, M.G.; Stasi, E.; Notarnicola, M.; Miraglia, C.; Nouvenne, A.; Meschi, T.; De’ Angelis, G.L.; Di Mario, F.; et al. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Biomed. 2018, 89, 60–75.
- Sugihara, K.; Morhardt, T.L.; Kamada, N. The Role of Dietary Nutrients in Inflammatory Bowel Disease. Front. Immunol. 2019, 9, 3183.
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283.
- Koleva, P.; Ketabi, A.; Valcheva, R.; Gänzle, M.G.; Dieleman, L.A. Chemically defined diet alters the protective properties of fructo-oligosaccharides and isomalto-oligosaccharides in HLA-B27 transgenic rats. PLoS ONE 2014, 9, e111717.
- Wright, R.; Truelove, S.C. A Controlled Therapeutic Trial of Various Diets in Ulcerative Colitis. BMJ 1965, 2, 138–141.
- Strisciuglio, C.; Giannetti, E.; Martinelli, M.; Sciorio, E.; Staiano, A.; Miele, E. Does cow’s milk protein elimination diet have a role on induction and maintenance of remission in children with ulcerative colitis? Acta Paediatr. 2013, 102, e273–e278.
- Bhattacharyya, S.; Shumard, T.; Xie, H.; Dodda, A.; Varady, K.A.; Feferman, L.; Halline, A.G.; Goldstein, J.L.; Hanauer, S.B.; Tobacman, J.K. A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity. Nutr. Healthy Aging 2017, 4, 181–192.
- Lim, H.-S.; Kim, S.-K.; Hong, S.-J. Food Elimination Diet and Nutritional Deficiency in Patients with Inflammatory Bowel Disease. Clin. Nutr. Res. 2018, 7, 48–55.
- Fahoum, L.; (Moscovici), A.J.; David, S.; Shaoul, R.; Rozen, G.; Meyron-Holtz, E.G.; Lesmes, U.; Meyron-Holtz, E.G. Digestive fate of dietary carrageenan: Evidence of interference with digestive proteolysis and disruption of gut epithelial function. Mol. Nutr. Food Res. 2017, 61, 1600545.
- Weiner, M.L.; McKim, J.M. Comment on ₜRevisiting the carrageenan controversy: Do we really understand the digestive fate and safety of carrageenan in our foods?. Food Funct. 2019, 10, 1760–1762.
- Candy, S.; Borok, G.; Wright, J.P.; Boniface, V.; Goodman, R. The value of an elimination diet in the management of patients with ulcerative colitis. South Afr. Med. J. 1995, 85, 1176–1179.
- Kyaw, M.H.; Moshkovska, T.; Mayberry, J. A prospective, randomized, controlled, exploratory study of comprehensive dietary advice in ulcerative colitis: Impact on disease activity and quality of life. Eur. J. Gastroenterol. Hepatol. 2014, 26, 910–917.
- Pedersen, N.; Ankersen, D.V.; Felding, M.; Wachmann, H.; Végh, Z.; Molzen, L.; Burisch, J.; Andersen, J.R.; Munkholm, P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 3356–3366.
- Jian, L.; Anqi, H.; Gang, L.; Litian, W.; Yanyan, X.; Mengdi, W.; Tong, L. Food Exclusion Based on IgG Antibodies Alleviates Symptoms in Ulcerative Colitis: A Prospective Study. Inflamm. Bowel Dis. 2018, 24, 1918–1925.
- Limketkai, B.N.; Iheozor-Ejiofor, Z.; Gjuladin-Hellon, T.; Parian, A.; Matarese, L.E.; Bracewell, K.; Macdonald, J.K.; Gordon, M.; Mullin, G.E. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 2019, 2, CD012.
- Haskey, N.; Gibson, D.L. An Examination of Diet for the Maintenance of Remission in Inflammatory Bowel Disease. Nutrients 2017, 9, 259.
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, R.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 39–49.
This entry is offline, you can click here to edit this entry!
