Some wild-type and genetically modified bacteria can metabolize xylose through three different main pathways of metabolism: xylose isomerase pathway, oxidoreductase pathway, and non-phosphorylative pathway (including Weimberg and Dahms pathways). Two of the commercially interesting intermediates of these pathways are xylitol and xylonic acid, which can accumulate in the medium either through manipulation of the culture conditions or through genetic modification of the bacteria.
- D-xylose
- biorefinery
- circular economy
- sustainable processes
- metabolism in bacteria
1. Introduction
2. Major Routes of Xylose Transport and Metabolism in Bacteria
Xylose is a very common sugar in residual lignocellulosic biomass, being the second major sugar found in most lignocellulosic hydrolysates and the major sugar in hemicellulosic hydrolysates. For that reason, xylose is a very promising carbon source, and it makes sense to understand the fundamentals of the mechanism used by bacteria to metabolize xylose into high-value by-products.
2.1. Mechanisms of Xylose Transport
Regarding D-xylose, yeast and fungi can use facilitated diffusion or active transport, while bacteria tend to use active transport mechanisms [41]. These types of mechanisms are mediated by carrier proteins and, hence, exhibit the properties of specific inhibition, substrate specificity, and saturability. These processes enable sugar transportation against a concentration gradient at the expense of metabolic energy.
Bacteria species such as Bacillus, Clostridium, Escherichia coli, and Lactococcus use active transport for the uptake of xylose into the cell [41,44]. Usually, there are high- and low-affinity transporter routes. In E. coli, the most studied species, the high-affinity transporter (XylFGH) belongs to the ATP-binding cassette (ABC) family, while the low-affinity transporter (XlyE), a proton-coupled symporter, belongs to the major facilitator superfamily (MFS), with xylose transport being driven by a proton motive force [45,46,47,48]. The low-affinity transport mechanism is also present in Bacillus megaterium, Bacillus subtilis, Lactobacillus brevis, Salmonella typhimurium, Tetragenococcus halophila, and some ruminal bacteria [47,49,50,51,52,53,54,55]. The ABC transporter is more efficient concerning xylose uptake and comprises a D-xylose-binding protein XylF, the membrane permease XylH, and the ATP-binding protein XylG [48,56]. This system is also present in bacteria such as Clostridium, Geobacillus, or Thermoanaerobacter species [41,57,58,59]. However, this transport can be inhibited when other readily fermentable substrates, such as glucose, are present. Many microbial strains have a regulatory mechanism, carbon catabolite repression (CCR), mainly mediated by components of the phosphoenolpyruvate (PEP): carbohydrate phosphotransferase system (PTS), which prevents the expression of genes needed for catabolism of other carbon sources, namely pentoses, while the substrate that enables the fastest growth (normally glucose) is present [60,61]. Concerning E. coli, at least two mechanisms of xylose transport and metabolism repression were reported, including the XylR regulator and cyclic AMP (cAMP) receptor protein (CRP)-dependent control of Xyl genes and the presence of arabinose, since the transporters allow for the transportation of this sugar at lower efficiencies [62]. The presence of high levels of glucose leads to the dephosphorylation of the component EIIA of PTS, which becomes unable to activate the enzyme adenylate cyclase, originating low levels of cAMP. In contrast, when glucose levels drop, cAMP increases, activating CRP that, together with XylR (activated when bound by xylose), stimulate the operons xylFGH and xylAB, involved in xylose transport and metabolism [53]. These repressive mechanisms could bring difficulties in the utilization of lignocellulosic hydrolysates, where both sugars are present. In this kind of media, a diauxic growth is observed and the preferential substrate, usually glucose, is consumed first. When glucose is depleted in the culture medium and another non-repressive substrate such as xylose is present, there is a temporary cessation of growth and catabolic repression is then relieved.
3.2. Xylose Metabolic Network in Bacteria
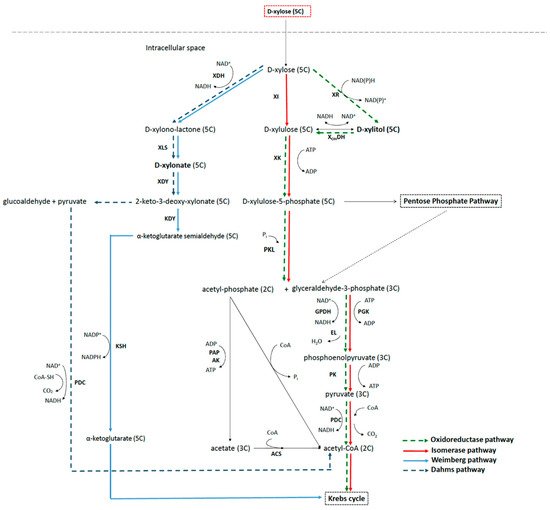
3.3. Metabolic Pathways to Xylitol and Xylonic Acid
3.3.1. Xylitol
Table 1 summarizes the contents of this section regarding a selection of bacterial strains able to convert xylose into xylitol.
| Strains | C-Source | Genetic Modification | Growth Conditions | Xylitol (g L−1) * |
Yxylitol/xylose (g g−1) |
Productivity (g L−1 h−1) * |
Ref. |
|---|---|---|---|---|---|---|---|
| Corynebacterium glutamicum Cg-ax3 |
arabinose glucose xylose |
Yes | Batch shake flask | 6.7 | n.a. | n.a. | [122] |
| Fed-batch shake flask | 31 | n.a. | 0.28 gg−1cdw h−1 | ||||
| acid pre-treated liquor of sorghum stover | Fed-batch shake flask | 27 | n.a. | 0.22 gg−1cdw h−1 | |||
| Corynebacterium sp. NRRL B 4247 | xylose | No | Shake flask | 1.7 | 0.57 | 0.071 | [108] |
| 6-phosphogluconate (source of NADPH) added to the medium Shake flask |
10 | n.a. | 0.067 | ||||
| Corynebacterium sp. no. 208 | xylose | No | 6-phosphogluconate (source of NADPH) was added to the medium Shake flask |
69 | n.a. | 0.21 | [123] |
| Enterobacter liquefaciens 553 |
xylose | No | Shake flask | 33 | n.a. | 0.35 | [110] |
| E. coli BL21(DE3) | xylose | Yes | Shake flask | 202 | 1.0 | 6.37 | [113] |
| Escherichia coli IS5-d | xylose and glucose | Yes | 5 L Batch STR | 110 | n.a. | 3.06 | [112] |
| Escherichia coli IS5-M | corncob hemicellulosic hydrolysate and 24 g L−1 corn steep liquor | Yes | 15 L Fed-batch STR | 144 | n.a. | 1.84 | [112] |
| Escherichia coli HK402 | xylose and glucose | Yes | 15 L Fed-batch STR | 172 | >0.95 | 1.57 | [112] |
| detoxified hemicellulosic hydrolysate and glucose | 150 | >0.95 | 1.40 | ||||
| Escherichia coli WZ51 | detoxified hemicellulosic hydrolysate | Yes | 15 L Fed-batch STR | 132 | 0.95 | 2.09 | [114] |
| Mycobacterium smegmatis | xylose | No | immobilized D-xylose isomerase from Bacillus coagulans and immobilized M. smegmatis Shake flask |
5 g | 0.80 | n.a. | [106] |
| Paraburkholderia sacchari DSM 17165 |
xylose | No | 2 L Fed-batch STR | 17 | n.a. | 0.39 | [60] |
| Paraburkholderia sacchari DSM 17165 |
xylose | No | 2 L Fed-batch STR | 70 | 0.39 | 0.50 | [80] |
3.3.2. Xylonic Acid
Table 2 gathers information on various examples of bacterial strains able to convert xylose into xylonic acid.
| Strains | C-Source | Genetic Modification | Growth Conditions | Xylonic Acid (g L−1) * |
Yxylonic acid/xylose (g g−1) |
Productivity (g L−1 h−1) * |
Ref. |
|---|---|---|---|---|---|---|---|
| Corynebacterium glutamicum ATCC13032 | xylose | Yes | Shake flask | 50.7 | 0.76 | 0.42 | [136] |
| Corynebacterium glutamicum ATCC31831 | rice straw hydrolysate after dilute sulfuric acid pretreatment | Yes | Shake flask | 42.9 | 1.1 | 0.37 | [136] |
| xylose | 56.3 | 0.84 | 0.47 | ||||
| Escherichia coli BL21 |
xylose | Yes | Shake flask | 9.1 | 1.10 | 0.45 | [141] |
| 2 L Batch STR | 6.9 | 0.89 | 0.11 | ||||
| Escherichia coli W3110 |
xylose and glucose | Yes | Shake flask | 5.1 | 0.51 | 0.084 | [134] |
| 5 L Fed-batch STR | 39.2 | 0.98 | 1.09 | ||||
| Escherichia coli BL21 |
xylose and glycerol | Yes | 5 L Fed-batch STR | 27.3 | n.a. | 1.8 | [135] |
| Gluconobacter oxydans ATCC 621 |
xylose | No | 3 L Batch STR | 109 | 0.95 | 2.5 | [127] |
| steamed and enzymatically hydrolyzed birchwood | 12.4 | 0.50 | n.a. | ||||
| Gluconobacter oxydans DSM 2003 |
corn stover hydrolysate after dry dilute acid pretreatment | No | 3 L Batch STR | 38.9 | 0.9 | n.a. | [139] |
| Gluconobacter oxydans DSM 2003 |
xylose | No | 3 L Batch STR | 66.4 | n.a. | 5.5 | [142] |
| Gluconobacter oxydans NL71 |
xylose | No | Compressed oxygen-supplied sealed stirred tank reactor (COS-SSTR); pure oxygen supply | 586.3 | 0.95 | 4.7 | [138] |
| corn stover diluted sulfuric acid hydrolysates without detoxification | 143.9 | 0.97 | 1.0 | ||||
| Gluconobacter oxydans NL71 |
xylose in distillation stillage of cellulosic ethanol fermentation broth |
No | COS-SSTR; fed-batch addition of xylose with cell-recycling | 1813 g in 6-fold cell recycling; 1 L culture medium | n.a. | 16.8 g h−1 in 108 h | [140] |
| Gluconobacter oxydans NL71 |
corn stover hydrolysate after dry diluted acid pretreatment | No | Two-stage fermentation in a 3 L COS-SSTR bioreactor with cell recycling | 167.4 g from 1 kg corn stover | 0.97 | 3.7 | [143] |
| Gluconobacter oxydans ATCC 621 |
xylose | No | Fed-batch bioreactor; Immobilized whole-cells; pressurized pure oxygen supply followed by electrodialysis acid chamber (POA-SSB-OE) | 329.2 g xylonic acid | n.a. | 7.1 g h−1 in 48 h | [144] |
| Klebsiella pneumoniae (modified) |
bamboo hydrolysate | Yes | Fed-batch cultivations | 103 | 0.98 | n.a. | [129] |
| Paraburkholderia sacchari DSM 17165 |
xylose | No | 2 L Fed-batch STR xylose as carbon source; high dissolved oxygen concentration |
150 | 0.85 | 1.5 | [80] |
| xylose and glucose | 2 L Fed-batch STR high dissolved oxygen concentration |
390 | 1.1 | 6.0 |
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/app11178112
