Mirizzi syndrome is a rare condition caused by the compression of the common hepatic duct due to stones located in the cystic duct or the neck of the gallbladder, which causes obstruction of the extrahepatic biliary tract, what is most commonly presented as jaundice and upper abdominal pain. Mirizzi syndrome occurs approximately in 0.05-4% of patients undergiong cholecystectomy. Prolonged inflammation caused by the stones impacted in the cystic duct or the neck of the gallbladder may lead to advanced stages of Mirizzi syndrome and the formation of a cholecystocholedochal fistula or even a cholecystoenteric fistula. Diagnosis is made upon the symptoms, laboratory results and imaging techniques such as ultrasonography, computed tomography, magnetic resonance imaging or endoscopic retrograde choleangiopancreatography (ERCP), which is considered as the golden standard. However, the preoperative diagnosis is difficult and a large part of all cases is diagnosed intraoperatively. Management of Mirizzi syndrome is mostly surgical, but early stages of the syndrome can be treated with the use of ERCP.
- classification
- ultrasonography (US)
- computed tomography (CT)
- magnetic resonance cholangiopancreatography (MRCP)
1. Introduction
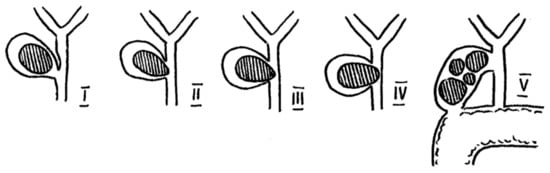
2. Classification
| Authors | McSherry et al.-1982 | Csendes et al.-1989 and Complemented in 2008 | Beltrán et al.-2012 | Payá-Llorente et al.-2017 | Nagakawa et al.-1997 |
|---|---|---|---|---|---|
| Classification | Type I—external compression of the bile duct | Type I—external compression of the bile duct | Type I—external compression of the bile duct | Type 1—external compression of the bile duct | Type I—external compression of the bile duct |
| Type II—cholecystobiliary fistula | Type II—cholecystobiliary fistula—up to 1/3 of the bile duct wall erosion | Type IIa—cholecystobiliary fistula involving <50% of the bile duct diameter | Type 2—cholecystobiliary fistula involving <50% of the bile duct diameter | Type II—cholecystobiliary fistula | |
| Type III—cholecystobiliary fistula—up to 2/3 of the bile duct wall erosion | |||||
| Type IV—cholecystobiliary fistula—complete destruction of the bile duct wall and fusion with gallbladder | Type IIb—cholecystobiliary fistula involving >50% of the bile duct diameter | Type 3—cholecystobiliary fistula involving >50% of the bile duct diameter | |||
| Type Va—cholecystoenteric fistula | Type IIIa—cholecystoenteric fistula | Subtypes describing cholecystoenteric fistula: A-no fistula/B-fistula without gallstone ileus/C-fistula with gallstone ileus | Type III—gallstones in the cystic duct and common hepatic duct confluence | ||
| Type Vb—cholecystoenteric fistula with gallstone ileus | Type IIIb—cholecystoenteric fistula with gallstone ileus | Type IV—stricture without stones (e.g., due to cholecystitis) |
3. Symptoms, laboratory resulst and imaging
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics11091660
References
- Fátima Senra; Lalin Navaratne; Asunción Acosta; Alberto Martínez-Isla; Laparoscopic management of type II Mirizzi syndrome. Surgical Endoscopy 2020, 34, 2303-2312, 10.1007/s00464-019-07316-6.
- Mohammed H.A. Al-Akeely; Mohammed K. Alam; Hayan Abdulrahman Bismar; Kamran Khalid; Ibrahim Al-Teimi; Nasser Faleh Al-Dossary; Mirizzi Syndrome: Ten Years Experience from a Teaching Hospital in Riyadh. World Journal of Surgery 2005, 29, 1687-1692, 10.1007/s00268-005-0100-3.
- Ashish Chawla; Jerome Irai Bosco; Tze Chwan Lim; Sivasubramanian Srinivasan; Hui Seong Teh; Jagadish Narayana Shenoy; Imaging of acute cholecystitis and cholecystitis-associated complications in the emergency setting. Singapore Medical Journal 2015, 56, 438-444, 10.11622/smedj.2015120.
- Rozina Mithani; Wayne H. Schwesinger; Juliane Bingener; Kenneth R. Sirinek; Glenn W. W. Gross; The Mirizzi Syndrome: Multidisciplinary Management Promotes Optimal Outcomes. Journal of Gastrointestinal Surgery 2007, 12, 1022-1028, 10.1007/s11605-007-0305-x.
- Hang Chen; Ernest Amos Siwo; Megan Khu; Yu Tian; Current trends in the management of Mirizzi Syndrome. Medicine 2018, 97, e9691, 10.1097/md.0000000000009691.
- Bader Shirah; Hamza Shirah; Khalid B Albeladi; Mirizzi syndrome: necessity for safe approach in dealing with diagnostic and treatment challenges. Annals of Hepato-Biliary-Pancreatic Surgery 2017, 21, 122-130, 10.14701/ahbps.2017.21.3.122.
- Ashok Kumar; Ganesan Senthil; Anand Prakash; Anu Behari; Rajneesh Kumar Singh; Vinay Kumar Kapoor; Rajan Saxena; Mirizzi's syndrome: lessons learnt from 169 patients at a single center. Korean Journal of Hepato-Biliary-Pancreatic Surgery 2016, 20, 17-22, 10.14701/kjhbps.2016.20.1.17.
- Yunfeng Cui; Yong Liu; Zhonglian Li; Erpeng Zhao; Hongtao Zhang; NaiQiang Cui; Appraisal of diagnosis and surgical approach for Mirizzi syndrome. ANZ Journal of Surgery 2012, 82, 708-713, 10.1111/j.1445-2197.2012.06149.x.
- Nisar A. Wani; Naseer A. Khan; Asif I. Shah; Abdul Q. Khan; Post-cholecystectomy Mirizzi′s syndrome: Magnetic resonance cholangiopancreatography demonstration. Saudi Journal of Gastroenterology : Official Journal of the Saudi Gastroenterology Association 2010, 16, 295-8, 10.4103/1319-3767.70620.
- Ahmad H. M. Nassar; Mahmoud K. Nassar; Ines C. Gil; Hwei J. Ng; Ahmad M. Yehia; One-session laparoscopic management of Mirizzi syndrome: feasible and safe in specialist units. Surgical Endoscopy 2020, 35, 3286-3295, 10.1007/s00464-020-07765-4.
- Ibrarullah, M.; Mishra, T.; Das, A.P; Mirizzi syndrome. The Indian Journal of Surgery 2008, 70, 281–287, 10.1007/s12262-008-0084-y.
- J. Sánchez Beorlequi; R. Cabezali Sánchez; E. Monsalve Laguna; P. Soriano Gil-Albarellos; N. Moreno de Marcos; Nuevas posibilidades diagnósticas y terapéuticas en el síndrome de Mirizzi. Anales de Medicina Interna 2007, 24, 281-284, 10.4321/s0212-71992007000600006.
- Pablo Acquafresca; Mariano Palermo; Luis Blanco; Rafael García; Francisco Tarsitano; [Mirizzi Syndrome: Prevalence, diagnosis and treatment].. Acta gastroenterologica Latinoamericana 2014, 44, 323–328, .
- Karen L.M. Tung; Chung N. Tang; Eric C.H. Lai; George P.C. Yang; Oliver C.Y. Chan; Michael K.W. Li; Robot-assisted Laparoscopic Approach of Management for Mirizzi Syndrome. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2013, 23, e17-e21, 10.1097/sle.0b013e3182724f9f.
- Mauricio Gonzalez-Urquijo; Gerardo Gil-Galindo; Mario Rodarte-Shade; Mirizzi syndrome from type I to Vb: a single center experience. Turkish Journal of Surgery 2020, 36, 399-404, 10.47717/turkjsurg.2020.4676.
- A. Rohatgi; K. K. Singh; Mirizzi syndrome: laparoscopic management by subtotal cholecystectomy. Surgical Endoscopy 2006, 20, 1477-1481, 10.1007/s00464-005-0623-6.
- Marvin B. Corlette; Biliobiliary Fistula. Archives of Surgery 1975, 110, 377-383, 10.1001/archsurg.1975.01360100019004.
- McSherry, C.K.; Ferstenberg, H.; Virshup, H; The Mirizzi syndrome : suggested classification and surgical therapy. Surg Gastroenterol. 1982, 1, 219–225, .
- A Csendes; J Carlos Diaz; P Burdiles; F Maluenda; O Nava; Mirizzi syndrome and cholecystobiliary fistula: A unifying classification. British Journal of Surgery 1989, 76, 1139-1143, 10.1002/bjs.1800761110.
- Marcelo A. Beltran; Attila Csendes; Karina S. Cruces; The Relationship of Mirizzi Syndrome and Cholecystoenteric Fistula: Validation of a Modified Classification. World Journal of Surgery 2008, 32, 2237-2243, 10.1007/s00268-008-9660-3.
- T Nagakawa; T Ohta; M Kayahara; K Ueno; I Konishi; H Sanada; I Miyazaki; A new classification of Mirizzi syndrome from diagnostic and therapeutic viewpoints.. Hepatogastroenterology 1997, 44, 63–67, .
- Cesar A. Solis-Caxaj; Mirizzi Syndrome: Diagnosis, Treatment and a Plea for a Simplified Classification. World Journal of Surgery 2009, 33, 1783-1784, 10.1007/s00268-009-9929-1.
- Marcelo A Beltrán; Mirizzi syndrome: History, current knowledge and proposal of a simplified classification. World Journal of Gastroenterology 2012, 18, 4639-4650, 10.3748/wjg.v18.i34.4639.
- Carmen Payá-Llorente; Antonio Vázquez-Tarragón; Antonio Alberola-Soler; Aleix Martínez-Pérez; Elías Martínez-López; Sandra Santarrufina-Martínez; Inmaculada Ortiz-Tarín; Ernesto Armañanzas-Villena; Mirizzi syndrome: a new insight provided by a novel classification. Annals of Hepato-Biliary-Pancreatic Surgery 2017, 21, 67-75, 10.14701/ahbps.2017.21.2.67.
- Ya Feng Ji; Yu Gao; Min Xie; The use of different pathology classification systems in preoperative imaging of Mirizzi syndrome.. Archives of Medical Science 2019, 15, 1288-1293, 10.5114/aoms.2019.87131.
- Young Erben; Luis A. Benavente-Chenhalls; John M. Donohue; Florencia G. Que; Michael L. Kendrick; Kaye M. Reid-Lombardo; Michael B. Farnell; David M. Nagorney; Diagnosis and Treatment of Mirizzi Syndrome: 23-Year Mayo Clinic Experience. Journal of the American College of Surgeons 2011, 213, 114-119, 10.1016/j.jamcollsurg.2011.03.008.
- Jaques Waisberg; Adriano Corona; Isaac Abreu; José Francisco De Matos Farah; Renato Arioni Lupinacci; Fábio Schmidt Goffi; Benign obstruction of the common hepatic duct (Mirizzi syndrome): diagnosis and operative management. Arquivos de Gastroenterologia 2005, 42, 13-18, 10.1590/s0004-28032005000100005.
- D. Gomez; S.H. Rahman; G.J. Toogood; K.R. Prasad; J.P.A. Lodge; P.J. Guillou; K.V. Menon; Mirizzi's syndrome – results from a large western experience. HPB 2006, 8, 474-479, 10.1080/13651820600840082.
- Jose B. Lledó; Sebastian M. Barber; Jose C. Ibañez; Antonio G. Torregrosa; R. Lopez-Andujar; Update on the Diagnosis and Treatment of Mirizzi Syndrome in Laparoscopic Era. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2014, 24, 495-501, 10.1097/sle.0000000000000079.
- Wei Ming Seah; Ye Xin Koh; Peng Chung Cheow; Pierce K.H. Chow; Chung Yip Chan; Ser Yee Lee; London L.P.J. Ooi; Alexander Y.F. Chung; Brian K.P. Goh; A Retrospective Review of the Diagnostic and Management Challenges of Mirizzi Syndrome at the Singapore General Hospital. Digestive Surgery 2017, 35, 491-497, 10.1159/000484256.
- Naduthottam Palaniswami Kamalesh; Kurumboor Prakash; Kaniyarakal Pramil; Thaliyachira Deepak George; Aikot Sylesh; Ponnambathayil Shaji; Laparoscopic approach is safe and effective in the management of Mirizzi syndrome. Journal of Minimal Access Surgery 2015, 11, 246-50, 10.4103/0972-9941.140216.
- Theegala L.V.D. Prasad; Ashok Kumar; Sadiq S. Sikora; Rajan Saxena; Vinay K. Kapoor; Mirizzi syndrome and gallbladder cancer. Journal of Hepato-Biliary-Pancreatic Surgery 2006, 13, 323-326, 10.1007/s00534-005-1072-2.
- Xie-Qun Xu; Tao Hong; Bing-Lu Li; Wei Liu; Xiao-Dong He; Chao-Ji Zheng; Mirizzi Syndrome: Our Experience with 27 Cases in PUMC Hospital. Chinese Medical Sciences Journal 2013, 28, 172-177, 10.1016/s1001-9294(13)60044-9.
- Sushil K. Ahlawat; Rohit Singhania; Firas H. Al-Kawas; Mirizzi syndrome. Current Treatment Options in Gastroenterology 2007, 10, 102-110, 10.1007/s11938-007-0062-7.
- T. Wehrmann; A. Riphaus; K. Martchenko; S. Kokabpick; H. Pauka; N. Stergiou; M. B. Frenz; Intraductal ultrasonography in the diagnosis of Mirizzi syndrome. Endoscopy 2006, 38, 717-722, 10.1055/s-2006-944524.
- Davlatov, S.; Rakhmanov, K.; Qurbonov, N.; Vafayeva, I.; Abduraxmanov, D; Current State of The Problem Treatment of Mirizzi Syndrome (Literature Review). International Journal of Pharmaceutical Research 2020, 12, 1931–1939, 10.31838/ijpr/2020.sp2.340.
- Winnie A. Mar; Andrew M. Shon; Yang Lu; Jonathan H. Yu; Senta M. Berggruen; Grace Guzman; Charles E. Ray; Frank Miller; Charles E. Ray Jr.; Imaging spectrum of cholangiocarcinoma: role in diagnosis, staging, and posttreatment evaluation. Abdominal Radiology 2016, 41, 553-567, 10.1007/s00261-015-0583-9.
- Jean-Marc Dumonceau; Myriam Delhaye; Nicolas Charette; Annarita Farina; Challenging biliary strictures: pathophysiological features, differential diagnosis, diagnostic algorithms, and new clinically relevant biomarkers - part 1. Therapeutic Advances in Gastroenterology 2020, 13, 553–567, 10.1177/1756284820927292.
- Hrishikesh P. Salgaonkar; Rachana D. Tataria; Gaurav Maheshwari; Premashish J. Halder; Mirizzi's syndrome: A scoring system for preoperative diagnosis. Saudi Journal of Gastroenterology 2018, 24, 274-281, 10.4103/sjg.SJG_6_18.
- Mario Testini; Lucia Ilaria Sgaramella; Giuseppe Massimiliano De Luca; Alessandro Pasculli; Angela Gurrado; Antonio Biondi; Giuseppe Piccinni; Management of Mirizzi Syndrome in Emergency. Journal of Laparoendoscopic & Advanced Surgical Techniques 2017, 27, 28-32, 10.1089/lap.2016.0315.
- Eun Joo Yun; Chul Soon Choi; Dae Young Yoon; Young Lan Seo; Suk Ki Chang; Joo Seop Kim; Ji Young Woo; Combination of Magnetic Resonance Cholangiopancreatography and Computed Tomography for Preoperative Diagnosis of the Mirizzi Syndrome. Journal of Computer Assisted Tomography 2009, 33, 636-640, 10.1097/rct.0b013e31817710d5.
- Jiannan Zhao; Ying Fan; Shuodong Wu; Safety and feasibility of laparoscopic approaches for the management of Mirizzi syndrome: a systematic review. Surgical Endoscopy 2020, 34, 4717-4726, 10.1007/s00464-020-07785-0.
- Hirdaya Hulas Nag; Phani Kumar Nekarakanti; Laparoscopic versus open surgical management of patients with Mirizzi's syndrome: A comparative study. Journal of Minimal Access Surgery 2020, 16, 215, 10.4103/jmas.jmas_33_19.
- Hua Zhong; Jian-Ping Gong; Mirizzi Syndrome: Experience in Diagnosis and Treatment of 25 Cases. The American Surgeon 2012, 78, 61-65, 10.1177/000313481207800136.
- Gustavo Salgado-Garza; Pamela Hernandez-Arriaga; Mauricio Gonzalez-Urquijo; José Antonio Díaz-Elizondo; Eduardo Flores-Villalba; Javier Rojas-Méndez; Mario Rodarte-Shade; Single-operator cholangioscopy and electrohydraulic lithotripsy for the treatment of Mirizzi syndrome. Annals of Medicine and Surgery 2021, 62, 274-277, 10.1016/j.amsu.2021.01.031.
- Susumu Tazuma; Michiaki Unno; Yoshinori Igarashi; Kazuo Inui; Kazuhisa Uchiyama; Masahiro Kai; Toshio Tsuyuguchi; Hiroyuki Maguchi; Toshiyuki Mori; Koji Yamaguchi; et al. Evidence-based clinical practice guidelines for cholelithiasis 2016. Journal of Gastroenterology 2016, 52, 276-300, 10.1007/s00535-016-1289-7.
